Perlecan regulates pericyte dynamics in the maintenance and repair of the blood-brain barrier
- PMID: 31541017
- PMCID: PMC6781430
- DOI: 10.1083/jcb.201807178
Perlecan regulates pericyte dynamics in the maintenance and repair of the blood-brain barrier
Abstract
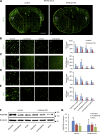
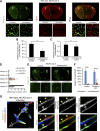
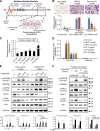
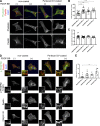
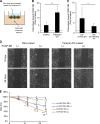
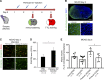
Ischemic stroke causes blood-brain barrier (BBB) breakdown due to significant damage to the integrity of BBB components. Recent studies have highlighted the importance of pericytes in the repair process of BBB functions triggered by PDGFRβ up-regulation. Here, we show that perlecan, a major heparan sulfate proteoglycan of basement membranes, aids in BBB maintenance and repair through pericyte interactions. Using a transient middle cerebral artery occlusion model, we found larger infarct volumes and more BBB leakage in conditional perlecan (Hspg2)-deficient (Hspg2 - / - -TG) mice than in control mice. Control mice showed increased numbers of pericytes in the ischemic lesion, whereas Hspg2 - / - -TG mice did not. At the mechanistic level, pericytes attached to recombinant perlecan C-terminal domain V (perlecan DV, endorepellin). Perlecan DV enhanced the PDGF-BB-induced phosphorylation of PDGFRβ, SHP-2, and FAK partially through integrin α5β1 and promoted pericyte migration. Perlecan therefore appears to regulate pericyte recruitment through the cooperative functioning of PDGFRβ and integrin α5β1 to support BBB maintenance and repair following ischemic stroke.
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Figures


References
-
- Bix G., Fu J., Gonzalez E.M., Macro L., Barker A., Campbell S., Zutter M.M., Santoro S.A., Kim J.K., Hook M., et al. . 2004. Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through alpha2beta1 integrin. J. Cell Biol. 166:97–109. 10.1083/jcb.200401150 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

