Homomeric GluA2(R) AMPA receptors can conduct when desensitized
- PMID: 31541113
- PMCID: PMC6754398
- DOI: 10.1038/s41467-019-12280-9
Homomeric GluA2(R) AMPA receptors can conduct when desensitized
Abstract
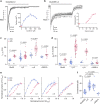
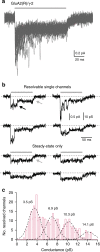
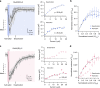
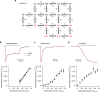
Desensitization is a canonical property of ligand-gated ion channels, causing progressive current decline in the continued presence of agonist. AMPA-type glutamate receptors (AMPARs), which mediate fast excitatory signaling throughout the brain, exhibit profound desensitization. Recent cryo-EM studies of AMPAR assemblies show their ion channels to be closed in the desensitized state. Here we present evidence that homomeric Q/R-edited AMPARs still allow ions to flow when the receptors are desensitized. GluA2(R) expressed alone, or with auxiliary subunits (γ-2, γ-8 or GSG1L), generates large fractional steady-state currents and anomalous current-variance relationships. Our results from fluctuation analysis, single-channel recording, and kinetic modeling, suggest that the steady-state current is mediated predominantly by conducting desensitized receptors. When combined with crystallography this unique functional readout of a hitherto silent state enabled us to examine cross-linked cysteine mutants to probe the conformation of the desensitized ligand binding domain of functioning AMPAR complexes.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

