Effect of Oral Oseltamivir on Virological Outcomes in Low-risk Adults With Influenza: A Randomized Clinical Trial
- PMID: 31541242
- PMCID: PMC7245154
- DOI: 10.1093/cid/ciz634
Effect of Oral Oseltamivir on Virological Outcomes in Low-risk Adults With Influenza: A Randomized Clinical Trial
Abstract
Background: Duration of viral shedding is a determinant of infectivity and transmissibility, but few data exist about oseltamivir's ability to alter viral shedding.
Methods: From January 2012 through October 2017, a randomized, double-blinded multicenter clinical trial was conducted in adults aged 18-64 years at 42 sites in Thailand, the United States, and Argentina. Participants with influenza A or B and without risk factors for complications of influenza were screened for the study. Eligible participants were randomized to receive oseltamivir 75 mg or placebo twice daily for 5 days. The primary endpoint was the percentage of participants with virus detectable by polymerase chain reaction in nasopharyngeal swab at day 3.
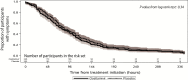
Results: Of 716 adults screened for the study, 558 were randomized, and 501 were confirmed to have influenza. Forty-six participants in the pilot study were excluded, and 449 of the 455 participants in the population for the primary analysis had day 3 viral shedding results. Ninety-nine (45.0%) of 220 participants in the oseltamivir arm had virus detected at day 3 compared with 131 (57.2%) of 229 participants in the placebo arm (absolute difference of -12.2% [-21.4%, -3.0%], P =; .010). The median time to alleviation of symptoms was 79.0 hours for the oseltamivir arm and 84.0 hours for the placebo arm (P =; .34) in those with confirmed influenza infection.
Conclusions: Oseltamivir decreased viral shedding in this low-risk population. However, in the population enrolled in this study, it did not significantly decrease the time to resolution of clinical symptoms.
Clinical trials registration: NCT01314911.
Keywords: influenza-like illness; respiratory virus; viral shedding.
Published by Oxford University Press for the Infectious Diseases Society of America 2019. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures
References
-
- Nicholson KG, Aoki FY, Osterhaus AD, et al. . Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet 2000; 355: 1845–50. - PubMed
-
- Treanor JJ, Hayden FG, Vrooman PS, et al. . Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA 2000; 283: 1016–24. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical