Triaging women with human papillomavirus infection and normal cytology or low-grade dyskaryosis: evidence from 10-year follow up of the ARTISTIC trial cohort
- PMID: 31541495
- PMCID: PMC6916371
- DOI: 10.1111/1471-0528.15957
Triaging women with human papillomavirus infection and normal cytology or low-grade dyskaryosis: evidence from 10-year follow up of the ARTISTIC trial cohort
Abstract
Objectives: To estimate long-term cervical intraepithelial neoplasia grade 3 (CIN3) risks associated with different triage strategies for human papillomavirus positive (HPV+) women with a view to reducing unnecessary referrals.
Design: The ARTISTIC trial cohort was recruited in Manchester in 2001-03 and was followed up for CIN3 and cancer notification through national registration until December 2015.
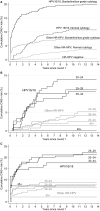
Results: The 10-year cumulative risk of CIN3+ was much higher for women with HPV16/18 infection (19.4%, 95% CI 15.8-23.8% with borderline/low-grade cytology and 10.7%, 95% CI 8.3-13.9% with normal cytology) than for those with other HPV types (7.3%, 95% CI 5.4-9.7% with borderline/low-grade cytology and 3.2%, 95% CI 2.2-4.5% with normal cytology). Among the 379 women with normal to low-grade cytology and new HPV infection, the 10-year cumulative CIN3+ risk was 2.9% (95% CI 1.6-5.2%).
Conclusions: The CIN3 risk is confined to women with persistent type-specific HPV so partial genotyping test assays identifying HPV16/18 as a minimum are essential for efficient risk stratification. Immediate referral to colposcopy for HPV+ women with borderline or low-grade cytology and referral after a year if still HPV+ with normal cytology may be unnecessary. Low-grade lesions can safely be retested to identify those with persistent HPV. Recall intervals of 1 year for HPV16/18 and 2 years for other high-risk HPVs are justified for women with normal cytology and might also be considered for women with borderline/low-grade cytology. The minimal risk of invasive cancer that has progressed beyond stage 1A must be weighed against the advantages for patients and the NHS of reducing the number of referrals to colposcopy.
Tweetable abstract: Cervical screening would be better for women and cheaper for the NHS if women with HPV and normal to low-grade cytology were retested after a year or two when many infections will have cleared.
Keywords: Cervical cancer; cervical intrapepithelial neoplasia grade 3; cervical screening; cytology; human papillomavirus; triage.
© 2019 The Authors. BJOG: An International Journal of Obstetrics and Gynaecology published by John Wiley & Sons Ltd on behalf of Royal College of Obstetricians and Gynaecologists.
Figures
Comment in
-
Beyond the scope of the HPV triage strategy - proposals for consideration.BJOG. 2020 Jan;127(1):69. doi: 10.1111/1471-0528.15983. Epub 2019 Nov 5. BJOG. 2020. PMID: 31605653 Free PMC article. No abstract available.
References
-
- Kitchener HC, Almonte M, Gilham C, Dowie R, Stoykova B, Sargent A, et al. ARTISTIC: a randomised trial of human papillomavirus (HPV) testing in primary cervical screening. Health Technol Assess 2009;13: 1–150, iii–iv. - PubMed
-
- Kitchener HC, Gilham C, Sargent A, Bailey A, Albrow R, Roberts C, et al. A comparison of HPV DNA testing and liquid based cytology over three rounds of primary cervical screening: extended follow up in the ARTISTIC trial. Eur J Cancer 2011;47:864–71. - PubMed
-
- Kitchener H, Canfell K, Gilham C, Sargent A, Roberts C, Desai M, et al. The clinical effectiveness and cost‐effectiveness of primary human papillomavirus cervical screening in England: extended follow‐up of the ARTISTIC randomised trial cohort through three screening rounds. Health Technol Assess 2014;18:1–196. - PMC - PubMed
-
- Ronco G, Dillner J, Elfstrom KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV‐based screening for prevention of invasive cervical cancer: follow‐up of four European randomised controlled trials. Lancet 2014;383:524–32. - PubMed
-
- Peto J, Gilham C, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. Lancet 2004;364:249–56. - PubMed