Pathogenic Tau Impairs Axon Initial Segment Plasticity and Excitability Homeostasis
- PMID: 31542321
- PMCID: PMC6880876
- DOI: 10.1016/j.neuron.2019.08.008
Pathogenic Tau Impairs Axon Initial Segment Plasticity and Excitability Homeostasis
Abstract
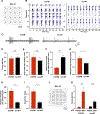
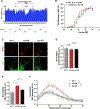
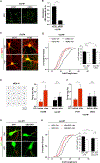
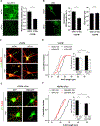
Dysregulation of neuronal excitability underlies the pathogenesis of tauopathies, including frontotemporal dementia (FTD) with tau inclusions. A majority of FTD-causing tau mutations are located in the microtubule-binding domain, but how these mutations alter neuronal excitability is largely unknown. Here, using CRISPR/Cas9-based gene editing in human pluripotent stem cell (iPSC)-derived neurons and isogenic controls, we show that the FTD-causing V337M tau mutation impairs activity-dependent plasticity of the cytoskeleton in the axon initial segment (AIS). Extracellular recordings by multi-electrode arrays (MEAs) revealed that the V337M tau mutation in human neurons leads to an abnormal increase in neuronal activity in response to chronic depolarization. Stochastic optical reconstruction microscopy of human neurons with this mutation showed that AIS plasticity is impaired by the abnormal accumulation of end-binding protein 3 (EB3) in the AIS submembrane region. These findings expand our understanding of how FTD-causing tau mutations dysregulate components of the neuronal cytoskeleton, leading to network dysfunction.
Keywords: EB3; FTD; axon initial segment; cytoskeleton; homeostasis; neuronal activity; tau.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Solving the Mysteries of Dementia: FTD Mutant Tau Impairs Structural Axon Initial Segment Plasticity.Neuron. 2019 Nov 6;104(3):429-430. doi: 10.1016/j.neuron.2019.10.021. Neuron. 2019. PMID: 31697915
References
-
- Ban H, Nishishita N, Fusaki N, Tabata T, Saeki K, Shikamura M, Takada N, Inoue M, Hasegawa M, Kawamata S, et al. (2011). Efficient generation of transgene-free human induced pluripotent stem cells (iPSCs) by temperature-sensitive Sendai virus vectors. Proc Natl Acad Sci U S A 108, 14234–14239. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

