Complement activation on neutrophils initiates endothelial adhesion and extravasation
- PMID: 31542608
- PMCID: PMC6815348
- DOI: 10.1016/j.molimm.2019.09.011
Complement activation on neutrophils initiates endothelial adhesion and extravasation
Abstract
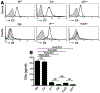
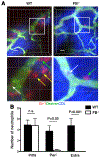
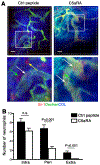
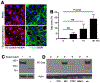
Neutrophils are essential to the pathogenesis of many inflammatory diseases. In the autoantibody-mediated K/BxN model of inflammatory arthritis, the alternative pathway (AP) of complement and Fc gamma receptors (FcγRs) are required for disease development while the classical pathway is dispensable. The reason for this differential requirement is unknown. We show that within minutes of K/BxN serum injection complement activation (CA) is detected on circulating neutrophils, as evidenced by cell surface C3 fragment deposition. CA requires the AP factor B and FcγRs but not C4, implying that engagement of FcγRs by autoantibody or immune complexes directly triggers AP C3 convertase assembly. The absence of C5 does not prevent CA on neutrophils but diminishes the upregulation of adhesion molecules. In vivo two-photon microscopy reveals that CA on neutrophils is critical for neutrophil extravasation and generation of C5a at the site of inflammation. C5a stimulates the release of neutrophil proteases, which contribute to the degradation of VE-cadherin, an adherens junction protein that regulates endothelial barrier integrity. C5a receptor antagonism blocks the extracellular release of neutrophil proteases, suppressing VE-cadherin degradation and neutrophil transendothelial migration in vivo. These results elucidate the AP-dependent intravascular neutrophil-endothelial interactions that initiate the inflammatory cascade in this disease model but may be generalizable to neutrophil extravasation in other inflammatory processes.
Keywords: C5a receptor; Complement; Fc gamma receptor; Inflammation; Neutrophils; Two-photon microscopy.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

