Effects of antihypertensives, lipid-modifying drugs, glycaemic control drugs and sodium bicarbonate on the progression of stages 3 and 4 chronic kidney disease in adults: a systematic review and meta-analysis
- PMID: 31542753
- PMCID: PMC6756484
- DOI: 10.1136/bmjopen-2019-030596
Effects of antihypertensives, lipid-modifying drugs, glycaemic control drugs and sodium bicarbonate on the progression of stages 3 and 4 chronic kidney disease in adults: a systematic review and meta-analysis
Abstract
Objective: To evaluate the effects of drug interventions that may modify the progression of chronic kidney disease (CKD) in adults with CKD stages 3 and 4.
Design: Systematic review and meta-analysis.
Methods: Searching MEDLINE, EMBASE, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, International Clinical Trials Registry Platform, Health Technology Assessment, Science Citation Index, Social Sciences Citation Index, Conference Proceedings Citation Index and Clinical Trials Register, from March 1999 to July 2018, we identified randomised controlled trials (RCTs) of drugs for hypertension, lipid modification, glycaemic control and sodium bicarbonate, compared with placebo, no drug or a drug from another class, in ≥40 adults with CKD stages 3 and/or 4, with at least 2 years of follow-up and reporting renal function (primary outcome), proteinuria, adverse events, maintenance dialysis, transplantation, cardiovascular events, cardiovascular mortality or all-cause mortality. Two reviewers independently screened citations and extracted data. For continuous outcomes, we used the ratio of means (ROM) at the end of the trial in random-effects meta-analyses. We assessed methodological quality with the Cochrane Risk of Bias Tool and confidence in the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework.
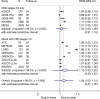
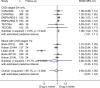
Results: We included 35 RCTs and over 51 000 patients. Data were limited, and heterogeneity varied. Final renal function (estimated glomerular filtration rate) was 6% higher in those taking glycaemic control drugs (ROM 1.06, 95% CI 1.02 to 1.10, I2=0%, low GRADE confidence) and 4% higher in those taking lipid-modifying drugs (ROM 1.04, 95% CI 1.00 to 1.08, I2=88%, very low GRADE confidence). For RCTs of antihypertensive drugs, there were no significant differences in renal function. Treatment with lipid-modifying drugs led to a 36% reduction in cardiovascular disease and 26% reduction in all-cause mortality.
Conclusions: Glycaemic control and lipid-modifying drugs may slow the progression of CKD, but we found no pooled evidence of benefit nor harm from antihypertensive drugs. However, given the data limitations, further research is needed to confirm these findings.
Prospero registration number: CRD42015017501.
Keywords: Cardiovascular events; Chronic renal failure; Drug treatment; Meta-analysis; Renal function.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: KST, JM, JKA and SF receive funding from the NIHR Programme for Applied Research. JYV receives funding from the NIHR Community Healthcare MIC, Nuffield Department of Primary Care Health Sciences and University of Oxford NIHR Diagnostic Evidence Co-operative (DEC). DSL receives funding from the NIHR Community Healthcare MIC. FDRH acknowledges his part-funding from the National Institute for Health Research (NIHR) School for Primary Care Research, the NIHR Collaboration for Leadership in Health Research and Care (CLAHRC) Oxford, the NIHR Oxford Biomedical Research Centre (BRC) (UHT), and the NIHR Oxford Medtech and In Vitro Diagnostics Co-operative (MIC). RP receives funding from the NIHR Oxford Biomedical Research Centre Programme, the NIHR Programme for Applied Research, the NIHR HPRU Gastrointestinal Infections Group, and the NIHR Diagnostic Evidence Co-operative (DEC).
Figures




References
-
- National Institute for Health and Care Excellence Chronic kidney disease: early identification and management of chronic kidney disease in adults in primary and secondary care (CG182. London: National Clinical Guideline Centre, 2014. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
