Appropriateness of initial dose of non-vitamin K antagonist oral anticoagulants in patients with non-valvular atrial fibrillation in the UK
- PMID: 31542760
- PMCID: PMC6756330
- DOI: 10.1136/bmjopen-2019-031341
Appropriateness of initial dose of non-vitamin K antagonist oral anticoagulants in patients with non-valvular atrial fibrillation in the UK
Erratum in
-
Correction: Appropriateness of initial dose of non-vitamin K antagonist oral anticoagulants in patients with non-valvular atrial fibrillation in the UK.BMJ Open. 2019 Oct 8;9(10):e031341corr1. doi: 10.1136/bmjopen-2019-031341corr1. BMJ Open. 2019. PMID: 31597654 Free PMC article. No abstract available.
Abstract
Objective: To evaluate the appropriateness of the initial prescribed daily dose of non-vitamin K antagonist oral anticoagulants (NOACs) according to label in patients with non-valvular atrial fibrillation (NVAF) in the UK.
Design: Population-based cross-sectional study.
Setting: UK primary care.
Population: 30 467 patients with NVAF and a first prescription for apixaban, dabigatran or rivaroxaban between January 2011 and December 2016.
Main outcome measures: Percentage of patients prescribed a NOAC dose according to the European Union (EU) labels (appropriately dosed), and not according to the EU labels (inappropriately dosed-including both underdosed and overdosed patients); percentage of patients prescribed an initial NOAC dose according to renal function status.
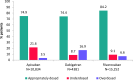
Results: A total of 15 252 (50.1%) patients started NOAC therapy on rivaroxaban, 10 834 (35.6%) on apixaban and 4381 (14.4%) on dabigatran. Among patients starting NOAC therapy on rivaroxaban, 17.3% were eligible to receive a reduced dose compared with 12.8% of patients starting on apixaban and 53.8% of patients starting on dabigatran. The majority of patients were prescribed an appropriate dose according to the EU labels: apixaban 74.9 %, dabigatran, 74.4%; rivaroxaban, 84.2%. Underdosing occurred in 21.6% (apixaban), 8.7% (dabigatran), 9.1% (rivaroxaban). Overdosing was more frequent for dabigatran (16.9%) than for rivaroxaban (6.6%) or apixaban (3.5%). There was a trend towards dose reduction with increasing renal impairment. Among patients with severe renal impairment, the majority received a reduced dose NOAC: apixaban, 91.1%, dabigatran, 80.0%, rivaroxaban, 83.0%.
Conclusion: Between 2011 and 2016, the majority of patients starting NOAC therapy in UK primary care were prescribed a daily dose in line with the approved EU drug label. Underdosing was more than twice as common among patients starting on apixaban than those starting on dabigatran or rivaroxaban. Research into the patient characteristics that may influence inappropriate underdosing of NOACs in UK primary care is warranted.
Keywords: Cardiac Epidemiology; EPIDEMIOLOGY; Thromboembolism.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: PV, YB, KS-W and BS are employees of Bayer AG (Germany), the funder of the study. GB is an employee of Bayer AB (Stockholm, Sweden). LR and SF are employees of Bayer PLC (Reading, UK). KS-W declares Bayer stocks. LR and SF declare shares in Bayer. LAGR, MM-P and AR work for the Spanish Centre for Pharmacoepidemiologic Research (Madrid, Spain), which has received research funding from Bayer AG. LAGR also declares honoraria for serving on advisory boards for Bayer AG.
Figures

Similar articles
-
Discontinuation of non-Vitamin K antagonist oral anticoagulants in patients with non-valvular atrial fibrillation: a population-based cohort study using primary care data from The Health Improvement Network in the UK.BMJ Open. 2019 Oct 18;9(10):e031342. doi: 10.1136/bmjopen-2019-031342. BMJ Open. 2019. PMID: 31630107 Free PMC article.
-
Apixaban for prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation in France: The PAROS cross-sectional study of routine clinical practice.Arch Cardiovasc Dis. 2019 Jun-Jul;112(6-7):400-409. doi: 10.1016/j.acvd.2019.02.003. Epub 2019 Apr 20. Arch Cardiovasc Dis. 2019. PMID: 31014991
-
Effectiveness and safety of reduced dose non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study.BMJ. 2017 Feb 10;356:j510. doi: 10.1136/bmj.j510. BMJ. 2017. PMID: 28188243 Free PMC article.
-
Direct comparative effectiveness and safety between non-vitamin K antagonist oral anticoagulants for stroke prevention in nonvalvular atrial fibrillation: a systematic review and meta-analysis of observational studies.Eur J Epidemiol. 2019 Feb;34(2):173-190. doi: 10.1007/s10654-018-0415-7. Epub 2018 Jun 8. Eur J Epidemiol. 2019. PMID: 29948370
-
Direct Oral Anticoagulants Versus Vitamin K Antagonists in Real-life Patients With Atrial Fibrillation. A Systematic Review and Meta-analysis.Rev Esp Cardiol (Engl Ed). 2019 Apr;72(4):305-316. doi: 10.1016/j.rec.2018.03.009. Epub 2018 Mar 30. Rev Esp Cardiol (Engl Ed). 2019. PMID: 29606361 English, Spanish.
Cited by
-
Inappropriate dosing of direct oral anticoagulants: findings from a clinical vignette study and physician survey.J Mark Access Health Policy. 2023 Oct 29;11(1):2267327. doi: 10.1080/20016689.2023.2267327. eCollection 2023. J Mark Access Health Policy. 2023. PMID: 37954532 Free PMC article.
-
Atrial fibrillation and oral anticoagulation in older people with frailty: a nationwide primary care electronic health records cohort study.Age Ageing. 2021 May 5;50(3):772-779. doi: 10.1093/ageing/afaa265. Age Ageing. 2021. PMID: 33951158 Free PMC article.
-
Determinants of label non-adherence to non-vitamin K oral anticoagulants in patients with newly diagnosed atrial fibrillation.Eur Heart J Open. 2022 Mar 29;2(3):oeac022. doi: 10.1093/ehjopen/oeac022. eCollection 2022 May. Eur Heart J Open. 2022. PMID: 35919339 Free PMC article.
-
Inappropriate Dosing of Direct Oral Anticoagulants in Patients with Atrial Fibrillation.Am J Cardiol. 2021 Apr 1;144:52-59. doi: 10.1016/j.amjcard.2020.12.062. Epub 2020 Dec 29. Am J Cardiol. 2021. PMID: 33385355 Free PMC article.
-
Hemorrhagic Coagulation Disorders and Ischemic Stroke: How to Reconcile Both?Neurol Int. 2023 Nov 30;15(4):1443-1458. doi: 10.3390/neurolint15040093. Neurol Int. 2023. PMID: 38132972 Free PMC article. Review.
References
-
- Ruigomez A, Brobert G, Vora P, et al. . Trends in use of rivaroxaban for prophylaxis and treatment in general practice in the United Kingdom between 2012 and 2015. Pharmacoepidemiol Drug Saf 2018;27.
-
- Fay MR, Martins JL, Czekay B. Oral anticoagulant prescribing patterns for stroke prevention in atrial fibrillation among general practitioners and cardiologists in three European countries. Eur Heart J 2016;37(Abstract Supplement):510. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
