Short-term outcomes of rapid initiation of antiretroviral therapy among HIV-positive patients: real-world experience from a single-centre retrospective cohort in Taiwan
- PMID: 31542770
- PMCID: PMC6756335
- DOI: 10.1136/bmjopen-2019-033246
Short-term outcomes of rapid initiation of antiretroviral therapy among HIV-positive patients: real-world experience from a single-centre retrospective cohort in Taiwan
Abstract
Objectives: Rapid initiation of antiretroviral therapy (ART) engenders faster viral suppression but with suboptimal rates of durable viral suppression and engagement in care, as reported by clinical trials in resource-limited settings. Real-world experience with rapid ART initiation remains limited in resource-rich settings.
Design: Retrospective cohort study.
Setting: A tertiary hospital in metropolitan Taipei, Taiwan.
Participants: We included 631 patients newly diagnosed as having HIV infection between March 2014 and July 2018.
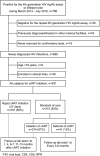
Main outcome measures: Rapid ART initiation was defined as starting ART within 7 days after HIV diagnosis confirmation. HIV diagnosis, ART initiation and viral suppression dates and clinical outcome data were collected by reviewing medical records. The rates of loss to follow-up (LTFU), engagement in care and virological rebound at 12 months were compared between patients with rapid ART initiation and those with standard initiation.
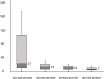
Results: Rapid ART initiation increased from 33.8% in 2014 to 68.3% in 2017, and the median interval between HIV diagnosis and viral suppression (HIV RNA load <200 copies/mL) decreased from 138 to 47 days. Patients with rapid ART initiation had a significantly higher rate of engagement in care at 12 months than did those with standard initiation (88.3% vs 79.0%; p=0.002). Patients aged <30 years had a higher risk of LTFU (HR: 2.19; 95% CI 1.20 to 3.98); and rapid ART initiation was associated with a lower risk of LTFU (HR: 0.41; 95% CI 0.24 to 0.83). Patients aged <30 years were more likely to acquire incident sexually transmitted infections (STIs) before achieving viral suppression.
Conclusions: Rapid ART initiation was associated with a higher rate of engagement in care at 12 months and shortened interval from diagnosis to HIV suppression. Delayed ART initiation may increase onwards HIV transmission considering the high rates of STIs.
Ethics approval: The study was approved by the Research Ethics Committee of National Taiwan University Hospital (Registration No. 201003112R).
Keywords: engagement in care; risk compensation; sexually transmitted infection; treatment as prevention; treatment cascade.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: C-CH received research support from Gilead Sciences, Merck and ViiV Healthcare and speaker honoraria from Gilead Sciences and ViiV Healthcare and served on the advisory boards for Gilead Sciences and ViiV Healthcare.
Figures


References
-
- WHO Guidelines Approved by the Guidelines Review Committee Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. 2015 Geneva: World Health Organization Copyright (c) World Health Organization, 2015. - PubMed
-
- Danel C, Moh R, Gabillard D, et al. . A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015;373:808–22. - PubMed
-
- O'Connor J, Vjecha MJ, Phillips AN, et al. . Effect of immediate initiation of antiretroviral therapy on risk of severe bacterial infections in HIV-positive people with CD4 cell counts of more than 500 cells per μL: secondary outcome results from a randomised controlled trial. The Lancet HIV 2017;4:e105–12. 10.1016/S2352-3018(16)30216-8 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
