Alternative dosing regimens for atezolizumab: an example of model-informed drug development in the postmarketing setting
- PMID: 31542806
- PMCID: PMC6820606
- DOI: 10.1007/s00280-019-03954-8
Alternative dosing regimens for atezolizumab: an example of model-informed drug development in the postmarketing setting
Abstract
Purpose: To determine the exposure-response (ER) relationships between atezolizumab exposure and efficacy or safety in patients with advanced non-small cell lung cancer (NSCLC) or urothelial carcinoma (UC) and to identify alternative dosing regimens.
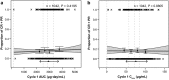
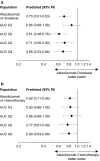
Methods: ER analyses were conducted using pooled NSCLC and UC data from phase 1 and 3 studies (PCD4989g, OAK, IMvigor211; ClinicalTrials.gov IDs, NCT01375842, NCT02008227, and NCT02302807, respectively). Objective response rate, overall survival, and adverse events were evaluated vs pharmacokinetic (PK) metrics. Population PK-simulated exposures for regimens of 840 mg every 2 weeks (q2w) and 1680 mg every 4 weeks (q4w) were compared with the approved regimen of 1200 mg every 3 weeks (q3w) and the maximum assessed dose (MAD; 20 mg/kg q3w). Phase 3 IMpassion130 (NCT02425891) data were used to validate the PK simulations for 840 mg q2w. Observed safety data were evaluated by exposure and body weight subgroups.
Results: No significant ER relationships were observed for safety or efficacy. Predicted exposures for 840 mg q2w and 1680 mg q4w were comparable to 1200 mg q3w and the MAD and consistent with observed PK data from IMpassion130. Observed safety was similar between patients with a Cmax above and below the predicted Cmax for 1680 mg q4w and between patients in the lowest and upper 3 body weight quartiles.
Conclusion: Atezolizumab regimens of 840 mg q2w and 1680 mg q4w are expected to have comparable efficacy and safety as the approved regimen of 1200 mg q3w, supporting their interchangeable use and offering patients greater flexibility.
Keywords: Atezolizumab; Exposure–response; PD-L1; Population pharmacokinetics.
Conflict of interest statement
K.M. Morrissey, H. Patel, R. Zhang, B. Wu, H.P. Chan, S. Girish, J.Y. Jin, H.R. Winter, and R. Bruno are employed by Genentech, Inc. M. Marchand is employed by Certara Strategic Consulting. A. Mecke is employed by F. Hoffmann-La Roche, Ltd.
Figures



Comment in
-
Alternative dosing regimens for atezolizumab: right dose, wrong frequency.Cancer Chemother Pharmacol. 2019 Dec;84(6):1153-1155. doi: 10.1007/s00280-019-03971-7. Epub 2019 Oct 19. Cancer Chemother Pharmacol. 2019. PMID: 31630223
References
-
- Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. - DOI - PMC - PubMed
-
- TECENTRIQ (atezolizumab) [package insert]. Genentech, Inc, South San Francisco, 2019
-
- TECENTRIQ (atezolizumab) [summary of product characteristics]. Roche Registration Limited, Welwyn Garden City, 2019
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

