Increased Vascular Contractility in Hypertension Results From Impaired Endothelial Calcium Signaling
- PMID: 31542964
- PMCID: PMC6791503
- DOI: 10.1161/HYPERTENSIONAHA.119.13791
Increased Vascular Contractility in Hypertension Results From Impaired Endothelial Calcium Signaling
Abstract
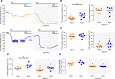
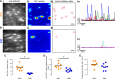
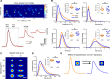
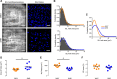
Endothelial cells line all blood vessels and are critical regulators of vascular tone. In hypertension, disruption of endothelial function alters the release of endothelial-derived vasoactive factors and results in increased vascular tone. Although the release of endothelial-derived vasodilators occurs in a Ca2+-dependent manner, little is known on how Ca2+ signaling is altered in hypertension. A key element to endothelial control of vascular tone is Ca2+ signals at specialized regions (myoendothelial projections) that connect endothelial cells and smooth muscle cells. This work describes disruption in the operation of this key Ca2+ signaling pathway in hypertension. We show that vascular reactivity to phenylephrine is increased in hypertensive (spontaneously hypertensive rat) when compared with normotensive (Wistar Kyoto) rats. Basal endothelial Ca2+ activity limits vascular contraction, but that Ca2+-dependent control is impaired in hypertension. When changes in endothelial Ca2+ levels are buffered, vascular contraction to phenylephrine increased, resulting in similar responses in normotension and hypertension. Local endothelial IP3(inositol trisphosphate)-mediated Ca2+ signals are smaller in amplitude, shorter in duration, occur less frequently, and arise from fewer sites in hypertension. Spatial control of endothelial Ca2+ signaling is also disrupted in hypertension: local Ca2+ signals occur further from myoendothelial projections in hypertension. The results demonstrate that the organization of local Ca2+ signaling circuits occurring at myoendothelial projections is disrupted in hypertension, giving rise to increased contractile responses.
Keywords: blood pressure; calcium; endothelial cells; hypertension; phenylephrine.
Figures

References
-
- Lawes CM, Vander Hoorn S, Rodgers A International Society of Hypertension. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–1518. doi: 10.1016/S0140-6736(08)60655-8. - PubMed
-
- Harris DM, Cohn HI, Pesant S, Eckhart AD. GPCR signalling in hypertension: role of GRKs. Clin Sci (Lond) 2008;115:79–89. doi: 10.1042/CS20070442. - PubMed
-
- Touyz RM. Molecular and cellular mechanisms in vascular injury in hypertension: role of angiotensin II. Curr Opin Nephrol Hypertens. 2005;14:125–131. - PubMed
-
- Coffman TM. Under pressure: the search for the essential mechanisms of hypertension. Nat Med. 2011;17:1402–1409. doi: 10.1038/nm.2541. - PubMed
-
- Touyz RM. New insights into mechanisms of hypertension. Curr Opin Nephrol Hypertens. 2012;21:119–121. doi: 10.1097/MNH.0b013e328350a50f. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

