Long-term immunity against yellow fever in children vaccinated during infancy: a longitudinal cohort study
- PMID: 31543249
- PMCID: PMC6892259
- DOI: 10.1016/S1473-3099(19)30323-8
Long-term immunity against yellow fever in children vaccinated during infancy: a longitudinal cohort study
Abstract
Background: A single dose of vaccine against yellow fever is routinely administered to infants aged 9-12 months under the Expanded Programme on Immunization, but the long-term outcome of vaccination in this age group is unknown. We aimed to evaluate the long-term persistence of neutralising antibodies to yellow fever virus following routine vaccination in infancy.
Methods: We did a longitudinal cohort study, using a microneutralisation assay to measure protective antibodies against yellow fever in Malian and Ghanaian children vaccinated around age 9 months and followed up for 4·5 years (Mali), or 2·3 and 6·0 years (Ghana). Healthy children with available day-0 sera, a complete follow-up history, and no record of yellow fever revaccination were included; children seropositive for yellow fever at baseline were excluded. We standardised antibody concentrations with reference to the yellow fever WHO International Standard.
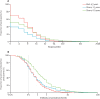
Findings: We included 587 Malian and 436 Ghanaian children vaccinated between June 5, 2009, and Dec 26, 2012. In the Malian group, 296 (50·4%, 95% CI 46·4-54·5) were seropositive (antibody concentration ≥0·5 IU/mL) 4·5 years after vaccination. Among the Ghanaian children, 121 (27·8%, 23·5-32·0) were seropositive after 2·3 years. These results show a large decrease from the proportions of seropositive infants 28 days after vaccination, 96·7% in Mali and 72·7% in Ghana, reported by a previous study of both study populations. The number of seropositive children increased to 188 (43·1%, 95% CI 38·5-47·8) in the Ghanaian group 6·0 years after vaccination, but this result might be confounded by unrecorded revaccination or natural infection with wild yellow fever virus during a 2011-12 outbreak in northern Ghana.
Interpretation: Rapid waning of immunity during the early years after vaccination of 9-month-old infants argues for a revision of the single-dose recommendation for this target population in endemic countries. The short duration of immunity in many vaccinees suggests that booster vaccination is necessary to meet the 80% population immunity threshold for prevention of yellow fever outbreaks.
Funding: Wellcome Trust.
Copyright © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures


Comment in
-
Are booster doses of yellow fever vaccine needed?Lancet Infect Dis. 2019 Dec;19(12):1275-1276. doi: 10.1016/S1473-3099(19)30411-6. Epub 2019 Sep 19. Lancet Infect Dis. 2019. PMID: 31543248 No abstract available.
-
Stablity of yellow fever virus neutralising antibody titres.Lancet Infect Dis. 2020 Feb;20(2):166-167. doi: 10.1016/S1473-3099(19)30703-0. Lancet Infect Dis. 2020. PMID: 32006501 No abstract available.
References
-
- WHO . Yellow fever fact sheet. World Health Organization; Geneva: 2019. https://www.who.int/news-room/fact-sheets/detail/yellow-fever
-
- Monath TP, Vasconcelos PF. Yellow fever. J Clin Virol. 2015;64:160–173. - PubMed
-
- WHO Vaccines and vaccination against yellow fever: WHO position paper, June 2013—recommendations. Vaccine. 2015;33:76–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

