NODULE INCEPTION Recruits the Lateral Root Developmental Program for Symbiotic Nodule Organogenesis in Medicago truncatula
- PMID: 31543454
- PMCID: PMC6839406
- DOI: 10.1016/j.cub.2019.09.005
NODULE INCEPTION Recruits the Lateral Root Developmental Program for Symbiotic Nodule Organogenesis in Medicago truncatula
Abstract
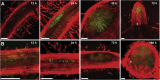
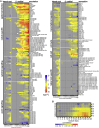
To overcome nitrogen deficiencies in the soil, legumes enter symbioses with rhizobial bacteria that convert atmospheric nitrogen into ammonium. Rhizobia are accommodated as endosymbionts within lateral root organs called nodules that initiate from the inner layers of Medicago truncatula roots in response to rhizobial perception. In contrast, lateral roots emerge from predefined founder cells as an adaptive response to environmental stimuli, including water and nutrient availability. CYTOKININ RESPONSE 1 (CRE1)-mediated signaling in the pericycle and in the cortex is necessary and sufficient for nodulation, whereas cytokinin is antagonistic to lateral root development, with cre1 showing increased lateral root emergence and decreased nodulation. To better understand the relatedness between nodule and lateral root development, we undertook a comparative analysis of these two root developmental programs. Here, we demonstrate that despite differential induction, lateral roots and nodules share overlapping developmental programs, with mutants in LOB-DOMAIN PROTEIN 16 (LBD16) showing equivalent defects in nodule and lateral root initiation. The cytokinin-inducible transcription factor NODULE INCEPTION (NIN) allows induction of this program during nodulation through activation of LBD16 that promotes auxin biosynthesis via transcriptional induction of STYLISH (STY) and YUCCAs (YUC). We conclude that cytokinin facilitates local auxin accumulation through NIN promotion of LBD16, which activates a nodule developmental program overlapping with that induced during lateral root initiation.
Keywords: CYTOKININ RESPONSE FACTOR; LATERAL ORGAN BOUNDARIES DOMAIN; Medicago truncatula; NODULE INCEPTION; YUCCA; auxin; endosymbiosis; lateral root/nodule organogenesis; nitrogen; rhizobia.
Copyright © 2019 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Oldroyd G.E., Murray J.D., Poole P.S., Downie J.A. The rules of engagement in the legume-rhizobial symbiosis. Annu. Rev. Genet. 2011;45:119–144. - PubMed
-
- Crespi M., Frugier F. De novo organ formation from differentiated cells: root nodule organogenesis. Sci. Signal. 2008;1:re11. - PubMed
-
- Plet J., Wasson A., Ariel F., Le Signor C., Baker D., Mathesius U., Crespi M., Frugier F. MtCRE1-dependent cytokinin signaling integrates bacterial and plant cues to coordinate symbiotic nodule organogenesis in Medicago truncatula. Plant J. 2011;65:622–633. - PubMed
-
- Liu J., Rutten L., Limpens E., van der Molen T., van Velzen R., Chen R., Chen Y., Geurts R., Kohlen W., Kulikova O. A Remote cis-Regulatory Region Is Required for NIN Expression in the Pericycle to Initiate Nodule Primordium Formation in Medicago truncatula. Plant Cell. 2019;31:68–83. - PMC - PubMed
-
- Marsh J.F., Rakocevic A., Mitra R.M., Brocard L., Sun J., Eschstruth A., Long S.R., Schultze M., Ratet P., Oldroyd G.E. Medicago truncatula NIN is essential for rhizobial-independent nodule organogenesis induced by autoactive calcium/calmodulin-dependent protein kinase. Plant Physiol. 2007;144:324–335. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

