Protein kinase C inhibitors override ZEB1-induced chemoresistance in HCC
- PMID: 31543517
- PMCID: PMC6755133
- DOI: 10.1038/s41419-019-1885-6
Protein kinase C inhibitors override ZEB1-induced chemoresistance in HCC
Abstract
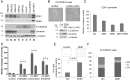
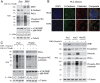
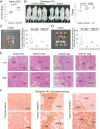
Epithelial-mesenchymal transition (EMT) is a process by which tumour cells lose epithelial characteristics, become mesenchymal and highly motile. EMT pathways also induce stem cell features and resistance to apoptosis. Identifying and targeting this pool of tumour cells is a major challenge. Protein kinase C (PKC) inhibition has been shown to eliminate breast cancer stem cells but has never been assessed in hepatocellular cancer (HCC). We investigated ZEB family of EMT inducer expression as a biomarker for metastatic HCC and evaluated the efficacy of PKC inhibitors for HCC treatment. We showed that ZEB1 positivity predicted patient survival in multiple cohorts and also validated as an independent biomarker of HCC metastasis. ZEB1-expressing HCC cell lines became resistant to conventional chemotherapeutic agents and were enriched in CD44high/CD24low cell population. ZEB1- or TGFβ-induced EMT increased PKCα abundance. Probing public databases ascertained a positive association of ZEB1 and PKCα expression in human HCC tumours. Inhibition of PKCα activity by small molecule inhibitors or by PKCA knockdown reduced viability of mesenchymal HCC cells in vitro and in vivo. Our results suggest that ZEB1 expression predicts survival and metastatic potential of HCC. Chemoresistant/mesenchymal HCC cells become addicted to PKC pathway and display sensitivity to PKC inhibitors such as UCN-01. Stratifying patients according to ZEB1 and combining UCN-01 with conventional chemotherapy may be an advantageous chemotherapeutic strategy.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

