Identifying Specific Subcellular Organelle Damage by Photosensitized Oxidations
- PMID: 31543705
- PMCID: PMC6747945
Identifying Specific Subcellular Organelle Damage by Photosensitized Oxidations
Abstract
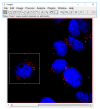
The search for conditions that maximize the outcome of Photodynamic Therapy (PDT) continues. Recent data indicate that PDT-induced cell death depends more on the specific intracellular location of the photosensitizer (PS) than on any other parameter. Indeed, knowledge of the PS intracellular location allows the establishment of clear relationships between the mechanism of cell death and the PDT efficacy. In order to determine the intracellular localization sites of a given PS, classical co-localization protocols, which are based in the comparison of the emissive profiles of organelle-specific probes to those of the PS, are usually performed. Since PSs are usually not efficient fluorophores, co-localization protocols require relatively high PS concentrations (micromolar range), distorting the whole proposal of the experiment, as high PS concentration means accumulation in many low-affinity sites. To overcome this difficulty, herein we describe a method that identifies PS intracellular localization by recognizing and quantifying the photodamage at intracellular organelles. We propose that irradiation protocols and characterization of major sites of photodamage results from many cycles of photosensitized oxidations, furnishing an integrated picture of the PS location. By comparing the results of protocols based in either method, we showed that the analysis of the damaged organelles can be conducted at optimal conditions (low PS concentrations), providing clear correlations with cell death mechanisms, which is not the case for the results obtained with co-localization protocols. Experiments using PSs that target either mitochondria or lysosomes were described and investigated in detail, showing that evaluating organelle damage is as simple as performing co-localization protocols.
Keywords: Photodamage; Photodynamic Therapy; intracellular localization; organelles; photosensitizer; specificity.
Figures







References
-
- Allison RR, Sibata CH, Downie GH, Cuenca RE. A clinical review of PDT for cutaneous malignancies. Photodiagn Photodyn Ther. 2006;3(4):214–26. - PubMed
-
- Hasan T, Ortel B, Moor AC, Pogue BW. Photodynamic therapy of cancer. In: Radiation Oncology. 1988. p. 43–55.
-
- Usuda J, Kato H, Okunaka T, Furukawa K, Yamada K, Suga Y, et al. Photodynamic Therapy (PDT) for Lung Cancers. J Thorac Oncol. 2006;1(5):489–93. - PubMed
-
- Babilas P, Schreml S, Landthaler M, Szeimies RM. Photodynamic therapy in dermatology: state-of-the-art. Photodermatol Photoimmunol Photomed. 2010;26(3):118–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
