GLUT4 Storage Vesicles: Specialized Organelles for Regulated Trafficking
- PMID: 31543708
- PMCID: PMC6747935
GLUT4 Storage Vesicles: Specialized Organelles for Regulated Trafficking
Abstract
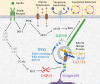
Fat and muscle cells contain a specialized, intracellular organelle known as the GLUT4 storage vesicle (GSV). Insulin stimulation mobilizes GSVs, so that these vesicles fuse at the cell surface and insert GLUT4 glucose transporters into the plasma membrane. This example is likely one instance of a broader paradigm for regulated, non-secretory exocytosis, in which intracellular vesicles are translocated in response to diverse extracellular stimuli. GSVs have been studied extensively, yet these vesicles remain enigmatic. Data support the view that in unstimulated cells, GSVs are present as a pool of preformed small vesicles, which are distinct from endosomes and other membrane-bound organelles. In adipocytes, GSVs contain specific cargoes including GLUT4, IRAP, LRP1, and sortilin. They are formed by membrane budding, involving sortilin and probably CHC22 clathrin in humans, but the donor compartment from which these vesicles form remains uncertain. In unstimulated cells, GSVs are trapped by TUG proteins near the endoplasmic reticulum - Golgi intermediate compartment (ERGIC). Insulin signals through two main pathways to mobilize these vesicles. Signaling by the Akt kinase modulates Rab GTPases to target the GSVs to the cell surface. Signaling by the Rho-family GTPase TC10α stimulates Usp25m-mediated TUG cleavage to liberate the vesicles from the Golgi. Cleavage produces a ubiquitin-like protein modifier, TUGUL, that links the GSVs to KIF5B kinesin motors to promote their movement to the cell surface. In obesity, attenuation of these processes results in insulin resistance and contributes to type 2 diabetes and may simultaneously contribute to hypertension and dyslipidemia in the metabolic syndrome.
Keywords: GLUT4 storage vesicle; GLUT4 trafficking; Unconventional secretion; Vasopressin inactivation; non-secretory exocytosis.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
