Inflammasomes in Colitis and Colorectal Cancer: Mechanism of Action and Therapies
- PMID: 31543710
- PMCID: PMC6747943
Inflammasomes in Colitis and Colorectal Cancer: Mechanism of Action and Therapies
Abstract
Colorectal cancer is a multifactorial disease and a leading cause of cancer-related deaths worldwide. Inflammation is a driver across multiple stages in the development of colorectal cancer. The inflammasome is a cytosolic multiprotein complex of the innate immune system central to the regulation of inflammation, pyroptosis, and other cellular processes important for maintaining gut homeostasis. Studies using mouse models of colitis and colitis-associated colorectal cancer have highlighted diverse and sometimes contrasting roles of inflammasomes in maintaining a balance between intestinal barrier function and the gut microbiota. In addition, persistent and/or dysregulated stimulation of inflammasome sensors finetune inflammation and tumorigenesis in the intestine. This review highlights the emerging role of inflammasome signaling in colitis and colitis-associated colorectal cancer. We also review the key mechanisms by which inflammasome signaling modulate inflammation and tumor development. Finally, we speculate the importance of using more tightly regulated experimental approaches to examine the role of gut microbiota in colorectal cancer.
Keywords: Inflammasomes; cancer; colitis; innate immunity; microbiome; microbiota; organelle; therapy.
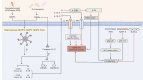
Figures
References
-
- Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA. et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524–48. - PMC - PubMed
-
- Brody H. Colorectal cancer. Nature. 2015;521(7551):S1. - PubMed
-
- Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med. 2015;372(15):1441–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
