Craving in Opioid Use Disorder: From Neurobiology to Clinical Practice
- PMID: 31543832
- PMCID: PMC6728888
- DOI: 10.3389/fpsyt.2019.00592
Craving in Opioid Use Disorder: From Neurobiology to Clinical Practice
Abstract
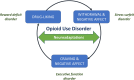
Opioid use disorder (OUD) is a major public health issue that has reached epidemic levels in some parts of the world. It is a chronic and complex neurobiological disease associated with frequent relapse to drug taking. Craving, defined as an overwhelmingly strong desire or need to use a drug, is a central component of OUD and other substance use disorders. In this review, we describe the neurobiological and neuroendocrine pathways that underpin craving in OUD and also focus on the importance of assessing and treating craving in clinical practice. Craving is strongly associated with patients returning to opioid misuse and is therefore an important treatment target to reduce the risk of relapse and improve patients' quality of life. Opioid agonist therapies (OAT), such as buprenorphine and methadone, can significantly reduce craving and relapse risk, and it is essential that patients are treated optimally with these therapies. There is also evidence to support the benefits of non-pharmacological approaches, such as cognitive behavioral therapy and mindfulness-based interventions, as supplementary treatments to opioid agonist therapies. However, despite the positive impact of these treatments on craving, many OUD patients continue to suffer with negative affect and dysphoria. There is a clear need for further studies to progress our understanding of the neurobiological basis of craving and addiction and to identify novel therapeutic strategies as well as to optimize the use of existing treatments to improve outcomes for the growing numbers of patients affected by OUD.
Keywords: addiction; buprenorphine; craving; methadone; negative affect; opioid.
Figures



Comment in
-
Commentary: Craving in Opioid Use Disorder: From Neurobiology to Clinical Practice.Front Psychiatry. 2021 Aug 9;12:615921. doi: 10.3389/fpsyt.2021.615921. eCollection 2021. Front Psychiatry. 2021. PMID: 34434123 Free PMC article. No abstract available.
References
-
- United Nations Office on Drugs and Crime (2017). World Drug Report 2017.
-
- American Academy of Pain Medicine, the American Pain Society, American Society of Addition Medicine Definitions related to the use of opioids for the treatment of pain. Wisconsin Med J (2001) 100:28–9. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

