The Role of Sex and Sex Hormones in Neurodegenerative Diseases
- PMID: 31544208
- PMCID: PMC7156855
- DOI: 10.1210/endrev/bnz005
The Role of Sex and Sex Hormones in Neurodegenerative Diseases
Abstract
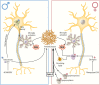
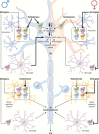
Neurodegenerative diseases (NDs) are a wide class of disorders of the central nervous system (CNS) with unknown etiology. Several factors were hypothesized to be involved in the pathogenesis of these diseases, including genetic and environmental factors. Many of these diseases show a sex prevalence and sex steroids were shown to have a role in the progression of specific forms of neurodegeneration. Estrogens were reported to be neuroprotective through their action on cognate nuclear and membrane receptors, while adverse effects of male hormones have been described on neuronal cells, although some data also suggest neuroprotective activities. The response of the CNS to sex steroids is a complex and integrated process that depends on (i) the type and amount of the cognate steroid receptor and (ii) the target cell type-either neurons, glia, or microglia. Moreover, the levels of sex steroids in the CNS fluctuate due to gonadal activities and to local metabolism and synthesis. Importantly, biochemical processes involved in the pathogenesis of NDs are increasingly being recognized as different between the two sexes and as influenced by sex steroids. The aim of this review is to present current state-of-the-art understanding on the potential role of sex steroids and their receptors on the onset and progression of major neurodegenerative disorders, namely, Alzheimer's disease, Parkinson's diseases, amyotrophic lateral sclerosis, and the peculiar motoneuron disease spinal and bulbar muscular atrophy, in which hormonal therapy is potentially useful as disease modifier.
Keywords: Alzheimer’s disease; Parkinson’s disease; amyotrophic lateral sclerosis; sex hormones; spinal and bulbar muscular atrophy.
© Endocrine Society 2019. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

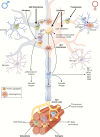


References
-
- Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77(1):10–18. - PubMed
-
- Paolicelli RC, Bolasco G, Pagani F, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. - PubMed
-
- Rodríguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A. Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience. 2016;323:170–182. - PubMed

