David vs. Goliath: The Structure, Function, and Clinical Prospects of Antibody Fragments
- PMID: 31544834
- PMCID: PMC6640713
- DOI: 10.3390/antib8020028
David vs. Goliath: The Structure, Function, and Clinical Prospects of Antibody Fragments
Abstract
Since the licensing of the first monoclonal antibody therapy in 1986, monoclonal antibodies have become the largest class of biopharmaceuticals with over 80 antibodies currently approved for a variety of disease indications. The development of smaller, antigen binding antibody fragments, derived from conventional antibodies or produced recombinantly, has been growing at a fast pace. Antibody fragments can be used on their own or linked to other molecules to generate numerous possibilities for bispecific, multi-specific, multimeric, or multifunctional molecules, and to achieve a variety of biological effects. They offer several advantages over full-length monoclonal antibodies, particularly a lower cost of goods, and because of their small size they can penetrate tissues, access challenging epitopes, and have potentially reduced immunogenicity. In this review, we will discuss the structure, production, and mechanism of action of EMA/FDA-approved fragments and of those in clinical and pre-clinical development. We will also discuss current topics of interest surrounding the potential use of antibody fragments for intracellular targeting and blood-brain barrier (BBB) penetration.
Keywords: ADC; BiTE®; ImmTAC®; Nanobody®; TandAb; V-NAR; antibody fragments; diabodies; domain antibodies; fab; scFv.
Conflict of interest statement
The authors declare no conflicts of interest. A.B. is a complementary worker on assignment at GSK. C.A.P. is a full time employee of GSK.
Figures
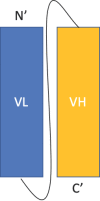
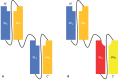




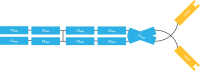
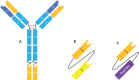




References
-
- Müller D., Kontermann R.E. Handbook of Therapeutic Antibodies. 2nd ed. Wiley-Blackwell; Hoboken, NJ, USA: 2014. Bispecific Antibodies.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

