Single-molecule visualization of human BLM helicase as it acts upon double- and single-stranded DNA substrates
- PMID: 31544923
- PMCID: PMC6868385
- DOI: 10.1093/nar/gkz810
Single-molecule visualization of human BLM helicase as it acts upon double- and single-stranded DNA substrates
Erratum in
-
Correction.Nucleic Acids Res. 2024 Jun 10;52(10):6093. doi: 10.1093/nar/gkae352. Nucleic Acids Res. 2024. PMID: 38686812 Free PMC article. No abstract available.
Abstract
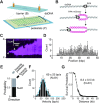
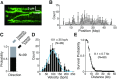
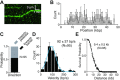
Bloom helicase (BLM) and its orthologs are essential for the maintenance of genome integrity. BLM defects represent the underlying cause of Bloom Syndrome, a rare genetic disorder that is marked by strong cancer predisposition. BLM deficient cells accumulate extensive chromosomal aberrations stemming from dysfunctions in homologous recombination (HR). BLM participates in several HR stages and helps dismantle potentially harmful HR intermediates. However, much remains to be learned about the molecular mechanisms of these BLM-mediated regulatory effects. Here, we use DNA curtains to directly visualize the activity of BLM helicase on single molecules of DNA. Our data show that BLM is a robust helicase capable of rapidly (∼70-80 base pairs per second) unwinding extensive tracts (∼8-10 kilobases) of double-stranded DNA (dsDNA). Importantly, we find no evidence for BLM activity on single-stranded DNA (ssDNA) that is bound by replication protein A (RPA). Likewise, our results show that BLM can neither associate with nor translocate on ssDNA that is bound by the recombinase protein RAD51. Moreover, our data reveal that the presence of RAD51 also blocks BLM translocation on dsDNA substrates. We discuss our findings within the context of potential regulator roles for BLM helicase during DNA replication and repair.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





Similar articles
-
Bloom syndrome helicase stimulates RAD51 DNA strand exchange activity through a novel mechanism.J Biol Chem. 2009 Sep 25;284(39):26349-59. doi: 10.1074/jbc.M109.029371. Epub 2009 Jul 24. J Biol Chem. 2009. PMID: 19632996 Free PMC article.
-
Single-Molecule Visualization of BLM-DNA2-Mediated DNA End Resection Using DNA Curtains.Methods Mol Biol. 2025;2881:195-211. doi: 10.1007/978-1-0716-4280-1_10. Methods Mol Biol. 2025. PMID: 39704945
-
Single-molecule visualization of human RECQ5 interactions with single-stranded DNA recombination intermediates.Nucleic Acids Res. 2021 Jan 11;49(1):285-305. doi: 10.1093/nar/gkaa1184. Nucleic Acids Res. 2021. PMID: 33332547 Free PMC article.
-
Bloom syndrome helicase in meiosis: Pro-crossover functions of an anti-crossover protein.Bioessays. 2017 Sep;39(9):10.1002/bies.201700073. doi: 10.1002/bies.201700073. Epub 2017 Aug 9. Bioessays. 2017. PMID: 28792069 Free PMC article. Review.
-
[Bloom syndrome].Nihon Rinsho. 2000 Jul;58(7):1460-6. Nihon Rinsho. 2000. PMID: 10921324 Review. Japanese.
Cited by
-
Condensin II interacts with BLM helicase in S phase to maintain genome stability.Commun Biol. 2025 Mar 25;8(1):492. doi: 10.1038/s42003-025-07916-0. Commun Biol. 2025. PMID: 40133469 Free PMC article.
-
Human RecQ Helicases in DNA Double-Strand Break Repair.Front Cell Dev Biol. 2021 Feb 25;9:640755. doi: 10.3389/fcell.2021.640755. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33718381 Free PMC article. Review.
-
ML216-Induced BLM Helicase Inhibition Sensitizes PCa Cells to the DNA-Crosslinking Agent Cisplatin.Molecules. 2022 Dec 12;27(24):8790. doi: 10.3390/molecules27248790. Molecules. 2022. PMID: 36557923 Free PMC article.
-
Single-molecule imaging of genome maintenance proteins encountering specific DNA sequences and structures.DNA Repair (Amst). 2023 Aug;128:103528. doi: 10.1016/j.dnarep.2023.103528. Epub 2023 Jun 24. DNA Repair (Amst). 2023. PMID: 37392578 Free PMC article. Review.
-
The multifaceted roles of DNA repair and replication proteins in aging and obesity.DNA Repair (Amst). 2021 Mar;99:103049. doi: 10.1016/j.dnarep.2021.103049. Epub 2021 Jan 21. DNA Repair (Amst). 2021. PMID: 33529944 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

