TRAIP drives replisome disassembly and mitotic DNA repair synthesis at sites of incomplete DNA replication
- PMID: 31545170
- PMCID: PMC6773462
- DOI: 10.7554/eLife.48686
TRAIP drives replisome disassembly and mitotic DNA repair synthesis at sites of incomplete DNA replication
Abstract
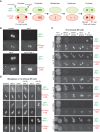
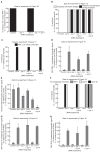
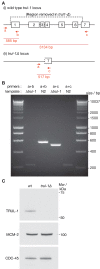
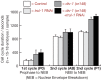
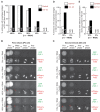
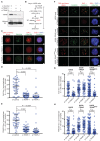
The faithful segregation of eukaryotic chromosomes in mitosis requires that the genome be duplicated completely prior to anaphase. However, cells with large genomes sometimes fail to complete replication during interphase and instead enter mitosis with regions of incompletely replicated DNA. These regions are processed in early mitosis via a process known as mitotic DNA repair synthesis (MiDAS), but little is known about how cells switch from conventional DNA replication to MiDAS. Using the early embryo of the nematode Caenorhabditis elegans as a model system, we show that the TRAIP ubiquitin ligase drives replisome disassembly in response to incomplete DNA replication, thereby providing access to replication forks for other factors. Moreover, TRAIP is essential for MiDAS in human cells, and is important in both systems to prevent mitotic segregation errors. Our data indicate that TRAIP is a master regulator of the processing of incomplete DNA replication during mitosis in metazoa.
Keywords: C. elegans; DNA replication; TRAIP; chromosomes; fragile site; gene expression; genome stability; human; mitosis; ubiquitin ligase.
© 2019, Sonneville et al.
Conflict of interest statement
RS, RB, SH, NM, IH, KL No competing interests declared
Figures






Similar articles
-
Mitotic CDK Promotes Replisome Disassembly, Fork Breakage, and Complex DNA Rearrangements.Mol Cell. 2019 Mar 7;73(5):915-929.e6. doi: 10.1016/j.molcel.2018.12.021. Mol Cell. 2019. PMID: 30849395 Free PMC article.
-
The Ubiquitin Ligase TRAIP: Double-Edged Sword at the Replisome.Trends Cell Biol. 2021 Feb;31(2):75-85. doi: 10.1016/j.tcb.2020.11.007. Epub 2020 Dec 11. Trends Cell Biol. 2021. PMID: 33317933 Free PMC article. Review.
-
CDK-driven phosphorylation of TRAIP is essential for mitotic replisome disassembly and MiDAS.Nucleic Acids Res. 2025 Jul 8;53(13):gkaf530. doi: 10.1093/nar/gkaf530. Nucleic Acids Res. 2025. PMID: 40637231 Free PMC article.
-
Mechanisms of eukaryotic replisome disassembly.Biochem Soc Trans. 2020 Jun 30;48(3):823-836. doi: 10.1042/BST20190363. Biochem Soc Trans. 2020. PMID: 32490508 Free PMC article. Review.
-
Mitotic replisome disassembly depends on TRAIP ubiquitin ligase activity.Life Sci Alliance. 2019 Apr 12;2(2):e201900390. doi: 10.26508/lsa.201900390. Print 2019 Apr. Life Sci Alliance. 2019. PMID: 30979826 Free PMC article.
Cited by
-
Short-term transcriptomic changes in the mouse neural tube induced by an acute alcohol exposure.Alcohol. 2023 Feb;106:1-9. doi: 10.1016/j.alcohol.2022.09.001. Epub 2022 Oct 4. Alcohol. 2023. PMID: 36202274 Free PMC article.
-
FAN1-mediated translesion synthesis and POLQ/HELQ-mediated end joining generate interstrand crosslink-induced mutations.Nat Commun. 2025 Mar 13;16(1):2495. doi: 10.1038/s41467-025-57764-z. Nat Commun. 2025. PMID: 40082407 Free PMC article.
-
The Fate of Two Unstoppable Trains After Arriving Destination: Replisome Disassembly During DNA Replication Termination.Front Cell Dev Biol. 2021 Jul 21;9:658003. doi: 10.3389/fcell.2021.658003. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34368118 Free PMC article. Review.
-
TTF2 promotes replisome eviction from stalled forks in mitosis.bioRxiv [Preprint]. 2024 Nov 30:2024.11.30.626186. doi: 10.1101/2024.11.30.626186. bioRxiv. 2024. PMID: 39651145 Free PMC article. Preprint.
-
Multiple DNA repair pathways contribute to MMS-induced post-replicative DNA synthesis in S. pombe.MicroPubl Biol. 2023 Oct 2;2023:10.17912/micropub.biology.000974. doi: 10.17912/micropub.biology.000974. eCollection 2023. MicroPubl Biol. 2023. PMID: 37854101 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

