Effect of omega-3 polyunsaturated fatty acid (n-3 PUFA) supplementation to lactating sows on growth and indicators of stress in the postweaned pig1,2
- PMID: 31545382
- PMCID: PMC6827406
- DOI: 10.1093/jas/skz300
Effect of omega-3 polyunsaturated fatty acid (n-3 PUFA) supplementation to lactating sows on growth and indicators of stress in the postweaned pig1,2
Abstract
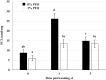
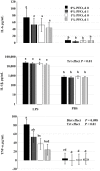
Dietary omega-3 polyunsaturated fatty acids (n-3 PUFA) are precursors for lipid metabolites that reduce inflammation. Two experiments were conducted to test the hypothesis that enriching the sow diet in n-3 PUFA during late gestation and throughout lactation reduces stress and inflammation and promotes growth in weaned pigs. A protected fish oil product (PFO; Gromega) was used to enrich the diet in n-3 PUFA. In the initial experiment, time-bred gilts were fed a gestation and lactation diet supplemented with 0% (control; n = 5), 0.25% (n = 4), 0.5% (n = 4), or 1% (n = 5) PFO from 101 ± 2 d of gestation to day 16 of lactation. Adding 1% PFO to the diet increased the n-3:n-6 PUFA ratio in colostrum and milk compared with controls (P = 0.05). A subsequent experiment was performed to determine whether supplementing the sow diet with 1% PFO improved growth and reduced circulating markers of acute inflammation and stress in the offspring. Plasma was harvested from piglets (16 per treatment group) on day 0 (d of weaning) and days 1 and 3 postweaning. Pigs from the 1% PFO treatment group weighed more (P = 0.03) on day 3 postweaning and had a greater (P ˂ 0.05) n-3:n-6 PUFA ratio in plasma on each day sampled compared with 0% PFO controls. There was an overall treatment effect on plasma total cortisol (P = 0.03) and haptoglobin (P = 0.04), with lesser concentrations in pigs on the 1% PFO diet. Plasma corticosteroid-binding globulin (CBG) concentrations were not different between treatment groups but were less (P ˂ 0.001) on days 1 and 3 when compared with day 0. The resultant free cortisol index [FCI (cortisol/CBG)] was less (P = 0.02) on days 1 and 3 for pigs from the 1% treatment group compared with the controls. An ex vivo lipopolysaccharide (LPS) challenge of whole blood collected on days 0 and 1 was used to determine whether 1% PFO attenuated release of inflammatory cytokines (IL-1β, IL-6, and TNF-α). Blood from pigs within the 1% PFO treatment group tended (P = 0.098) to have a lesser mean concentration of TNF-α in response to LPS compared with blood from controls. These results suggest that providing a PFO supplement as 1% of the diet to sows beginning in late gestation and during lactation can increase the n-3:n-6 PUFA ratio in their offspring, which may improve growth and reduce the acute physiological stress response in the pigs postweaning.
Keywords: fish oil; lactation; pig; sow; supplementation; weaning stress.
Published by Oxford University Press on behalf of the American Society of Animal Science 2019.
Figures





References
-
- Adcock R. J., Kattesh H. G., Roberts M. P., Carroll J. A., Saxton A. M., and Kojima C. J.. 2007. Temporal relationships between plasma cortisol, corticosteroid-binding globulin (CBG), and the free cortisol index (FCI) in pigs in response to adrenal stimulation or suppression. Stress 10:305–310. doi:10.1080/10253890701248020. - DOI - PubMed
-
- Burdick Sanchez N. C., Carroll J. A., Broadway P. R., Bass B. E., and Frank J. W.. 2019. Supplementation of a Lactobacillus acidophilus fermentation product can attenuate the acute phase response following a lipopolysaccharide challenge in weaned pigs. Animal 13:144–152. doi:10.1017/S1751731118001222. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

