Long-term efficacy and safety of sonidegib in patients with advanced basal cell carcinoma: 42-month analysis of the phase II randomized, double-blind BOLT study
- PMID: 31545507
- PMCID: PMC7318253
- DOI: 10.1111/bjd.18552
Long-term efficacy and safety of sonidegib in patients with advanced basal cell carcinoma: 42-month analysis of the phase II randomized, double-blind BOLT study
Abstract
Background: Basal cell carcinomas (BCCs) exhibit aberrant activation of the hedgehog pathway. Sonidegib is a hedgehog pathway inhibitor approved for the treatment of locally advanced BCC (laBCC) and metastatic BCC (mBCC) based on primary results of the BOLT study [Basal Cell Carcinoma Outcomes with LDE225 (sonidegib) Treatment].
Objectives: This is the final 42-month analysis of the BOLT study, evaluating the efficacy and safety of sonidegib.
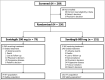
Methods: Adults with no prior hedgehog pathway inhibitor therapy were randomized in a 1 : 2 ratio to sonidegib 200 mg or 800 mg once daily. Treatment continued for up to 42 months or until disease progression, unacceptable toxicity, death, study termination or withdrawal of consent. The primary efficacy end point was the objective response rate (ORR) by central review, assessed at baseline; weeks 5, 9 and 17; then subsequently every 8 or 12 weeks during years 1 or 2, respectively. Safety end points included adverse event monitoring and reporting.
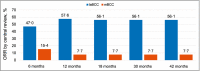
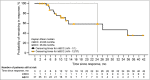
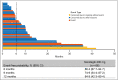
Results: The study enrolled 230 patients, 79 and 151 in the 200-mg and 800-mg groups, respectively, of whom 8% and 3.3% remained on treatment by the 42-month cutoff, respectively. The ORRs by central review were 56% [95% confidence interval (CI) 43-68] for laBCC and 8% (95% CI 0·2-36) for mBCC in the 200-mg group and 46·1% (95% CI 37·2-55·1) for laBCC and 17% (95% CI 5-39) for mBCC in the 800-mg group. No new safety concerns emerged.
Conclusions: Sonidegib demonstrated sustained efficacy and a manageable safety profile. The final BOLT results support sonidegib as a viable treatment option for laBCC and mBCC. What's already known about this topic? Basal cell carcinoma (BCC) is usually treatable with surgery or radiation therapy, but there are limited treatment options for patients with advanced BCC. Sonidegib, a hedgehog pathway inhibitor approved for the treatment of advanced BCC, demonstrated clinically relevant efficacy and manageable safety in prior analyses of the phase II randomized, double-blind BOLT study [Basal Cell Carcinoma Outcomes with LDE225 (sonidegib) Treatment]. What does this study add? This final 42-month analysis of BOLT is the longest follow-up available for a hedgehog pathway inhibitor. Clinically relevant efficacy results were sustained from prior analyses, with objective response rates by central review of the approved 200-mg daily dose of 56% in locally advanced BCC and 8% in metastatic BCC. No new safety concerns were raised. The results confirmed sonidegib as a viable long-term treatment option for patients with advanced BCC.
© 2019 The Authors. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Figures

Comment in
-
Hedgehog pathway inhibitors come of age.Br J Dermatol. 2020 Jun;182(6):1322-1323. doi: 10.1111/bjd.18737. Epub 2019 Dec 17. Br J Dermatol. 2020. PMID: 31849041 No abstract available.
References
-
- Asgari MM, Moffet HH, Ray GT, Quesenberry CP. Trends in basal cell carcinoma incidence and identification of high‐risk subgroups, 1998–2012. JAMA Dermatol 2015; 151:976–81. - PubMed
-
- Xiang F, Lucas R, Hales S, Neale R. Incidence of nonmelanoma skin cancer in relation to ambient UV radiation in white populations, 1978–2012: empirical relationships. JAMA Dermatol 2014; 150:1063–71. - PubMed
-
- Kim JYS, Kozlow JH, Mittal B et al Guidelines of care for the management of basal cell carcinoma. J Am Acad Dermatol 2018; 78:540–59. - PubMed
-
- Amici JM, Battistella M, Beylot‐Barry M et al Defining and recognising locally advanced basal cell carcinoma. Eur J Dermatol 2015; 25:586–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

