Phase II Trial of Response-Based Radiation Therapy for Patients With Localized CNS Nongerminomatous Germ Cell Tumors: A Children's Oncology Group Study
- PMID: 31545689
- PMCID: PMC6900864
- DOI: 10.1200/JCO.19.00701
Phase II Trial of Response-Based Radiation Therapy for Patients With Localized CNS Nongerminomatous Germ Cell Tumors: A Children's Oncology Group Study
Abstract
Purpose: Stratum 1 of ACNS1123 (ClinicalTrials.gov identifier: NCT01602666), a Children's Oncology Group phase II trial, evaluated efficacy of reduced-dose and volume of radiotherapy (RT) in children and adolescents with localized nongerminomatous germ cell tumors (NGGCTs). The primary objective was to evaluate the impact of reduced RT on progression-free survival (PFS) with a goal of preserving neurocognitive function.
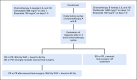
Patients and methods: Patients received six cycles of chemotherapy with carboplatin and etoposide alternating with ifosfamide and etoposide, as used in the Children's Oncology Group predecessor study (ACNS0122; ClinicalTrials.gov identifier: NCT00047320). Patients who achieved a complete response (CR) or partial response (PR) with or without second-look surgery were eligible for reduced RT, defined as 30.6 Gy whole ventricular field and 54 Gy tumor-bed boost, compared with 36 Gy craniospinal irradiation plus 54 Gy tumor-bed boost used in ACNS0122.
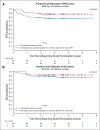
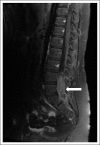
Results: A total of 107 eligible patients were enrolled. Median age was 10.98 years (range, 3.68 to 21.63) and 75% were male. Sixty-six of 107 (61.7%) achieved a CR or PR and proceeded to reduced RT. The 3-year PFS and overall survival and standard error values were 87.8% ± 4.04% and 92.4% ± 3.3% compared with 92% and 94.1%, respectively, in ACNS0122. There were 10 recurrences, prompting early study closure; however, after a retrospective central review, only disease in eight of 66 (12.1%) patients eligible for reduced RT subsequently progressed; six patients had distant spinal relapse alone and two had disease with combined local plus distant relapse. Serum and CSF α-fetoprotein and β-human chorionic gonadotropin levels were not associated with PFS.
Conclusion: Patients with localized NGGCT who achieved a CR or PR to chemotherapy and received reduced RT had encouraging PFS similar to patients in ACNS0122 who received full-dose craniospinal irradiation. However, the patterns of failure were distinct, with all patients having treatment failure in the spine.
Figures
References
-
- Crawford JR, Santi MR, Vezina G, et al. CNS germ cell tumor (CNSGCT) of childhood: Presentation and delayed diagnosis. Neurology. 2007;68:1668–1673. - PubMed
-
- Echevarría ME, Fangusaro J, Goldman S. Pediatric central nervous system germ cell tumors: A review. Oncologist. 2008;13:690–699. - PubMed
-
- Louis DN, Ohgaki H, Wiestler OD, et al. WHO Classification of Tumours of the Central Nervous System. 4th ed. Lyon, France: International Agency for Research on Cancer; 2007.
-
- Fujimaki T. Central nervous system germ cell tumors: Classification, clinical features, and treatment with a historical overview. J Child Neurol. 2009;24:1439–1445. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

