MiR-21 in Substance P-induced exosomes promotes cell proliferation and migration in human colonic epithelial cells
- PMID: 31545921
- PMCID: PMC6957364
- DOI: 10.1152/ajpgi.00043.2019
MiR-21 in Substance P-induced exosomes promotes cell proliferation and migration in human colonic epithelial cells
Abstract
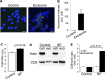
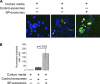
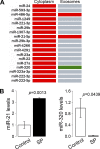
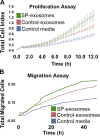
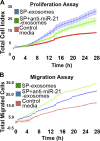
Exosomes are cellular vesicles involved in intercellular communication via their specialized molecular cargo, such as miRNAs. Substance P (SP), a neuropeptide/hormone, and its high-affinity receptor, NK-1R, are highly expressed during colonic inflammation. Our previous studies show that SP/NK-1R signaling stimulates differential miRNA expression and promotes colonic epithelial cell proliferation. In this study, we examined whether SP/NK-1R signaling regulates exosome biogenesis and exosome-miRNA cargo sorting. Moreover, we examined the role of SP/NK-1R signaling in exosome-regulated cell proliferation and migration. Exosomes produced by human colonic NCM460 epithelial cells overexpressing NK-1R (NCM460-NK1R) were isolated from culture media. Exosome abundance and uptake were assessed by Western blot analysis (abundance) and Exo-Green fluorescence microscopy (abundance and uptake). Cargo-miRNA levels were assessed by RT-PCR. Cell proliferation and migration were assessed using xCELLigence technology. Colonic epithelial exosomes were isolated from mice pretreated with SP for 3 days. Cell proliferation in vivo was assessed by Ki-67 staining. SP/NK-1R signaling in human colonic epithelial cells (in vitro) and mouse colons (in vivo) increased 1) exosome production, 2) the level of fluorescence in NCM460s treated with Exo-Green-labeled exosomes, and 3) the level of miR-21 in exosome cargo. Moreover, our results showed that SP/NK-1R-induced cell proliferation and migration are at least in part dependent on intercellular communication via exosomal miR-21 in vitro and in vivo. Our results demonstrate that SP/NK-1R signaling regulates exosome biogenesis and induces its miR-21 cargo sorting. Moreover, exosomal miR-21 promotes proliferation and migration of target cells.NEW & NOTEWORTHY Substance P signaling regulates exosome production in human colonic epithelial cells and colonic crypts in wild-type mice. MiR-21 is selectively sorted into exosomes induced by Substance P stimulation and promotes cell proliferation and migration in human colonocytes and mouse colonic crypts.
Keywords: NK-1R; Substance P; cell migration; cell proliferation; exosomes; miR-21; sorting.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Anderson MR, Pleet ML, Enose-Akahata Y, Erickson J, Monaco MC, Akpamagbo Y, Velluci A, Tanaka Y, Azodi S, Lepene B, Jones J, Kashanchi F, Jacobson S. Viral antigens detectable in CSF exosomes from patients with retrovirus associated neurologic disease: functional role of exosomes. Clin Transl Med 7: 24, 2018. doi:10.1186/s40169-018-0204-7. - DOI - PMC - PubMed
-
- Au Yeung CL, Co NN, Tsuruga T, Yeung TL, Kwan SY, Leung CS, Li Y, Lu ES, Kwan K, Wong KK, Schmandt R, Lu KH, Mok SC. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun 7: 11150, 2016. doi:10.1038/ncomms11150. - DOI - PMC - PubMed
-
- Bakirtzi K, Hatziapostolou M, Karagiannides I, Polytarchou C, Jaeger S, Iliopoulos D, Pothoulakis C. Neurotensin signaling activates microRNAs-21 and −155 and Akt, promotes tumor growth in mice, and is increased in human colon tumors. Gastroenterology 141: 1749–1761, 2011. doi:10.1053/j.gastro.2011.07.038. - DOI - PMC - PubMed
-
- Castagliuolo I, Keates AC, Qiu B, Kelly CP, Nikulasson S, Leeman SE, Pothoulakis C. Increased substance P responses in dorsal root ganglia and intestinal macrophages during Clostridium difficile toxin A enteritis in rats. Proc Natl Acad Sci USA 94: 4788–4793, 1997. doi:10.1073/pnas.94.9.4788. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

