Enteric neuron density correlates with clinical features of severe gut dysmotility
- PMID: 31545923
- PMCID: PMC6962493
- DOI: 10.1152/ajpgi.00199.2019
Enteric neuron density correlates with clinical features of severe gut dysmotility
Abstract
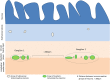
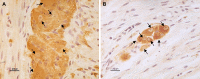
Gastrointestinal (GI) symptoms can originate from severe dysmotility due to enteric neuropathies. Current methods used to demonstrate enteric neuropathies are based mainly on classic qualitative histopathological/immunohistochemical evaluation. This study was designed to identify an objective morphometric method for paraffin-embedded tissue samples to quantify the interganglionic distance between neighboring myenteric ganglia immunoreactive for neuron-specific enolase, as well as the number of myenteric and submucosal neuronal cell bodies/ganglion in jejunal specimens of patients with severe GI dysmotility. Jejunal full-thickness biopsies were collected from 32 patients (22 females; 16-77 yr) with well-characterized severe dysmotility and 8 controls (4 females; 47-73 yr). A symptom questionnaire was filled before surgery. Mann-Whitney U test, Kruskal-Wallis coupled with Dunn's posttest and nonparametric linear regression tests were used for analyzing morphometric data and clinical correlations, respectively. Compared with controls, patients with severe dysmotility exhibited a significant increase in myenteric interganglionic distance (P = 0.0005) along with a decrease in the number of myenteric (P < 0.00001) and submucosal (P < 0.0004) neurons. A 50% reduction in the number of submucosal and myenteric neurons correlated with an increased interganglionic distance and severity of dysmotility. Our study proposes a relatively simple tool that can be applied for quantitative evaluation of paraffin sections from patients with severe dysmotility. The finding of an increased interganglionic distance may aid diagnosis and limit the direct quantitative analysis of neurons per ganglion in patients with an interganglionic distance within the control range.NEW & NOTEWORTHY Enteric neuropathies are challenging conditions characterized by a severe impairment of gut physiology, including motility. An accurate, unambiguous assessment of enteric neurons provided by quantitative analysis of routine paraffin sections may help to define neuropathy-related gut dysmotility. We showed that patients with severe gut dysmotility exhibited an increased interganglionic distance associated with a decreased number of myenteric and submucosal neurons, which correlated with symptoms and clinical manifestations of deranged intestinal motility.
Keywords: chronic intestinal pseudo-obstruction; enteric neuron cell count; interganglionic distance; severe gut dysmotility.
Conflict of interest statement
R. De Georgio has participated as a consultant for Shire, Sucampo, Coloplast, Kyowa Kirin International, and Takeda and received grant support from Shire and Takeda. These consultancies did not influence the content of this article. The other authors declare no conflict of interest.
Figures




References
-
- Bernard CE, Gibbons SJ, Gomez-Pinilla PJ, Lurken MS, Schmalz PF, Roeder JL, Linden D, Cima RR, Dozois EJ, Larson DW, Camilleri M, Zinsmeister AR, Pozo MJ, Hicks GA, Farrugia G. Effect of age on the enteric nervous system of the human colon. Neurogastroenterol Motil 21: 746–e46, 2009. doi:10.1111/j.1365-2982.2008.01245.x. - DOI - PMC - PubMed
-
- Bernardini N, Segnani C, Ippolito C, De Giorgio R, Colucci R, Faussone-Pellegrini MS, Chiarugi M, Campani D, Castagna M, Mattii L, Blandizzi C, Dolfi A. Immunohistochemical analysis of myenteric ganglia and interstitial cells of Cajal in ulcerative colitis. J Cell Mol Med 16: 318–327, 2012. doi:10.1111/j.1582-4934.2011.01298.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

