SIDT2 RNA Transporter Promotes Lung and Gastrointestinal Tumor Development
- PMID: 31546103
- PMCID: PMC6817685
- DOI: 10.1016/j.isci.2019.09.009
SIDT2 RNA Transporter Promotes Lung and Gastrointestinal Tumor Development
Abstract
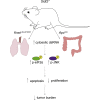
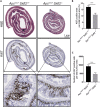
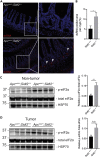
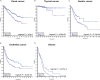
RNautophagy is a newly described type of selective autophagy whereby cellular RNAs are transported into lysosomes for degradation. This process involves the transmembrane protein SIDT2, which transports double-stranded RNA (dsRNA) across the endolysosomal membrane. We previously demonstrated that SIDT2 is a transcriptional target of p53, but its role in tumorigenesis, if any, is unclear. Unexpectedly, we show here that Sidt2-/- mice with concurrent oncogenic KrasG12D activation develop significantly fewer tumors than littermate controls in a mouse model of lung adenocarcinoma. Consistent with this observation, loss of SIDT2 also leads to enhanced survival and delayed tumor development in an Apcmin/+ mouse model of intestinal cancer. Within the intestine, Apcmin/+;Sidt2-/- mice display accumulation of dsRNA in association with increased phosphorylation of eIF2α and JNK as well as elevated rates of apoptosis. Taken together, our data demonstrate a role for SIDT2, and by extension RNautophagy, in promoting tumor development.
Keywords: Biological Sciences; Cancer; Cell Biology; Molecular Biology.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Lysosomal targeting of SIDT2 via multiple YxxΦ motifs is required for SIDT2 function in the process of RNautophagy.J Cell Sci. 2017 Sep 1;130(17):2843-2853. doi: 10.1242/jcs.202481. Epub 2017 Jul 19. J Cell Sci. 2017. PMID: 28724756
-
Cytosolic domain of SIDT2 carries an arginine-rich motif that binds to RNA/DNA and is important for the direct transport of nucleic acids into lysosomes.Autophagy. 2020 Nov;16(11):1974-1988. doi: 10.1080/15548627.2020.1712109. Epub 2020 Jan 16. Autophagy. 2020. PMID: 31944164 Free PMC article.
-
Lysosomal putative RNA transporter SIDT2 mediates direct uptake of RNA by lysosomes.Autophagy. 2016;12(3):565-78. doi: 10.1080/15548627.2016.1145325. Autophagy. 2016. PMID: 27046251 Free PMC article.
-
SIDT1 Localizes to Endolysosomes and Mediates Double-Stranded RNA Transport into the Cytoplasm.J Immunol. 2019 Jun 15;202(12):3483-3492. doi: 10.4049/jimmunol.1801369. Epub 2019 May 6. J Immunol. 2019. PMID: 31061008
-
The functions of SID1 transmembrane family, member 2 (Sidt2).FEBS J. 2023 Oct;290(19):4626-4637. doi: 10.1111/febs.16641. Epub 2022 Oct 14. FEBS J. 2023. PMID: 36176242 Review.
Cited by
-
Cryo-EM structures of human SID-1 transmembrane family proteins and implications for their low-pH-dependent RNA transport activity.Cell Res. 2024 Jan;34(1):80-83. doi: 10.1038/s41422-023-00893-1. Epub 2023 Nov 6. Cell Res. 2024. PMID: 37932445 Free PMC article. No abstract available.
-
Identification of Key Deregulated RNA-Binding Proteins in Pancreatic Cancer by Meta-Analysis and Prediction of Their Role as Modulators of Oncogenesis.Front Cell Dev Biol. 2021 Nov 29;9:713852. doi: 10.3389/fcell.2021.713852. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34912796 Free PMC article.
-
The emerging mechanisms and functions of microautophagy.Nat Rev Mol Cell Biol. 2023 Mar;24(3):186-203. doi: 10.1038/s41580-022-00529-z. Epub 2022 Sep 12. Nat Rev Mol Cell Biol. 2023. PMID: 36097284 Review.
-
Structure of the human systemic RNAi defective transmembrane protein 1 (hSIDT1) reveals the conformational flexibility of its lipid binding domain.Life Sci Alliance. 2024 Jun 26;7(9):e202402624. doi: 10.26508/lsa.202402624. Print 2024 Sep. Life Sci Alliance. 2024. PMID: 38925866 Free PMC article.
-
Effects of SIDT2 on the miR-25/NOX4/HuR axis and SIRT3 mRNA stability lead to ROS-mediated TNF-α expression in hydroquinone-treated leukemia cells.Cell Biol Toxicol. 2023 Oct;39(5):2207-2225. doi: 10.1007/s10565-022-09705-5. Epub 2022 Mar 18. Cell Biol Toxicol. 2023. PMID: 35302183
References
-
- Beck A., Fecher-Trost C., Wolske K., Philipp S.E., Flockerzi V., Wissenbach U. Identification of Sidt2 as a lysosomal cation-conducting protein. FEBS Lett. 2017;591:76–87. - PubMed
-
- Chang G., Yang R., Cao Y., Nie A., Gu X., Zhang H. Sidt2 is involved in the NAADP-mediated release of calcium from insulin secretory granules. J. Mol. Endocrinol. 2016;56:249–259. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

