High dose interleukin-2 (Aldesleukin) - expert consensus on best management practices-2014
- PMID: 31546315
- PMCID: PMC6889624
- DOI: 10.1186/s40425-014-0026-0
High dose interleukin-2 (Aldesleukin) - expert consensus on best management practices-2014
Abstract
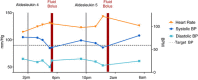
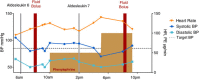
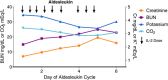
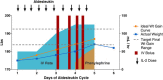
Interleukin-2 (IL-2) was historically one of the few treatments for adults with stage IV solid tumors that could produce complete responses (CRs) that were often durable for decades without further therapy. The majority of complete responders with metastatic renal cell carcinoma (mRCC) and metastatic melanoma (mM) could probably be classified as "cures". Recent publications have suggested improved efficacy, perhaps due to improved patient selection based on a better understanding of clinical features predicting outcomes. Guidelines for clinical management were established from experience at the National Cancer Institute (NCI) and an affiliation of institutions known as the Cytokine Working Group (CWG), who were among the first to utilize HD IL-2 treatment outside of the NCI. As new centers have opened, further management variations have emerged based upon center-specific experience, to optimize administration of IL-2 and provide high quality care for patients at each individual site. Twenty years of evolution in differing environments has led to a plethora of clinical experience and effective management approaches. The goal of this review is to summarize the spectrum of HD IL-2 treatment approaches, describing various effective strategies that incorporate newer adjunctive treatments for managing the side effects of IL-2 in patients with mRCC and mM. The goal for IL-2 therapy is typically to administer the maximum number of doses of IL-2 without putting the patient at unacceptable risk for severe, irreversible toxicity. This review is based upon a consensus meeting and includes guidelines on pre-treatment screening, criteria for administration and withholding doses, and defines consensus criteria for safe administration and toxicity management. The somewhat heterogeneous best practices of 2014 will be compared and contrasted with the guidelines provided in 2001 and the package inserts from 1992 and 1998.
Keywords: Clinical management; Cytokines; Interleukin-2; Melanoma; Renal cell carcinoma; Treatment guidelines.
Figures
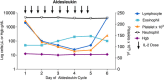
References
-
- Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, Seipp CA, Rogers-Freezer L, Morton KE, White DE, Liewehr DJ, Merino MJ, Rosenberg SA. Randomized study of high-dose and low-dose Interleukin-2 in patients with metastatic renal cell cancer. J Clin Oncol. 2003;21:3127–3132. doi: 10.1200/JCO.2003.02.122. - DOI - PMC - PubMed
-
- McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF, Kirkwood JM, Gordon MS, Sosman JA, Ernstoff MS, Tretter CPG, Urba WJ, Smith JW, Margolin KA, Mier JW, Gollob JA, Dutcher JP, Atkins MB. Randomized phase III trial of high dose Interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133–141. doi: 10.1200/JCO.2005.03.206. - DOI - PubMed
-
- Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Wiess G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, Paradise C, Kunkel L, Rosenberg SA. High dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. - PubMed
-
- Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6(Suppl 1):S11–S14. - PubMed
-
- Margolin KA, Rayner AA, Hawkins MJ, Atkins MB, Dutcher JP, Fisher RI, Weiss GR, Doroshow JH, Jaffe HS, Roper M. Interleukin-2 and lymphokine-activated killer cell therapy of solid tumors: analysis of toxicity and management guidelines. J Clin Oncol. 1989;7:486–498. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
