Efficient Epidermal Growth Factor Receptor Targeting Oligonucleotide as a Potential Molecule for Targeted Cancer Therapy
- PMID: 31546749
- PMCID: PMC6801465
- DOI: 10.3390/ijms20194700
Efficient Epidermal Growth Factor Receptor Targeting Oligonucleotide as a Potential Molecule for Targeted Cancer Therapy
Abstract
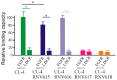
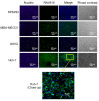
Epidermal growth factor receptor (EGFR) is associated with the progression of a wide range of cancers including breast, glioma, lung, and liver cancer. The observation that EGFR inhibition can limit the growth of EGFR positive cancers has led to the development of various EGFR inhibitors including monoclonal antibodies and small-molecule inhibitors. However, the reported toxicity and drug resistance greatly compromised the clinical outcome of such inhibitors. As a type of chemical antibodies, nucleic acid aptamer provides an opportunity to overcome the obstacles faced by current EGFR inhibitors. In this study, we have developed and investigated the therapeutic potential of a 27mer aptamer CL-4RNV616 containing 2'-O-Methyl RNA and DNA nucleotides. Our results showed that CL-4RNV616 not only displayed enhanced stability in human serum, but also effectively recognized and inhibited the proliferation of EGFR positive Huh-7 liver cancer, MDA-MB-231 breast cancer, and U87MG glioblastoma cells, with an IC50 value of 258.9 nM, 413.7 nM, and 567.9 nM, respectively. Furthermore, TUNEL apoptosis assay revealed that CL-4RNV616 efficiently induced apoptosis of cancer cells. In addition, clinical breast cancer biopsy-based immunostaining assay demonstrated that CL-4RNV616 had a comparable detection efficacy for EGFR positive breast cancer with commonly used commercial antibodies. Based on the results, we firmly believe that CL-4RNV616 could be useful in the development of targeted cancer therapeutics and diagnostics.
Keywords: Chemically-modified oligonucleotides; aptamers; nucleic acids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
A neutralizing RNA aptamer against EGFR causes selective apoptotic cell death.PLoS One. 2011;6(9):e24071. doi: 10.1371/journal.pone.0024071. Epub 2011 Sep 6. PLoS One. 2011. PMID: 21915281 Free PMC article.
-
Synergistic Targeting HER2 and EGFR with Bivalent Aptamer-siRNA Chimera Efficiently Inhibits HER2-Positive Tumor Growth.Mol Pharm. 2018 Nov 5;15(11):4801-4813. doi: 10.1021/acs.molpharmaceut.8b00388. Epub 2018 Oct 1. Mol Pharm. 2018. PMID: 30222359 Free PMC article.
-
Inhibition of cell proliferation by an anti-EGFR aptamer.PLoS One. 2011;6(6):e20299. doi: 10.1371/journal.pone.0020299. Epub 2011 Jun 8. PLoS One. 2011. PMID: 21687663 Free PMC article.
-
Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers.Pharmacol Res. 2019 Jan;139:395-411. doi: 10.1016/j.phrs.2018.11.014. Epub 2018 Nov 27. Pharmacol Res. 2019. PMID: 30500458 Review.
-
The epidermal growth factor receptor as a therapeutic target in glioblastoma multiforme and other malignant neoplasms.Anticancer Agents Med Chem. 2009 Jul;9(6):703-15. doi: 10.2174/187152009788680019. Anticancer Agents Med Chem. 2009. PMID: 19601750 Review.
Cited by
-
Aptamers: Novel Therapeutics and Potential Role in Neuro-Oncology.Cancers (Basel). 2020 Oct 9;12(10):2889. doi: 10.3390/cancers12102889. Cancers (Basel). 2020. PMID: 33050158 Free PMC article. Review.
-
Development of a CD63 Aptamer for Efficient Cancer Immunochemistry and Immunoaffinity-Based Exosome Isolation.Molecules. 2020 Nov 27;25(23):5585. doi: 10.3390/molecules25235585. Molecules. 2020. PMID: 33261145 Free PMC article.
-
The Application of Aptamer and Research Progress in Liver Disease.Mol Biotechnol. 2024 May;66(5):1000-1018. doi: 10.1007/s12033-023-01030-4. Epub 2024 Feb 2. Mol Biotechnol. 2024. PMID: 38305844 Free PMC article. Review.
-
Composites of Nucleic Acids and Boron Clusters (C2B10H12) as Functional Nanoparticles for Downregulation of EGFR Oncogene in Cancer Cells.Int J Mol Sci. 2021 May 4;22(9):4863. doi: 10.3390/ijms22094863. Int J Mol Sci. 2021. PMID: 34064412 Free PMC article.
-
Exosomes and exosome composite scaffolds in periodontal tissue engineering.Front Bioeng Biotechnol. 2024 Jan 18;11:1287714. doi: 10.3389/fbioe.2023.1287714. eCollection 2023. Front Bioeng Biotechnol. 2024. PMID: 38304105 Free PMC article. Review.
References
-
- Wang M., Zhao J., Zhang L.M., Li H., Yu J.P., Ren X.B., Wang C.L. Combined Erlotinib and Cetuximab overcome the acquired resistance to epidermal growth factor receptors tyrosine kinase inhibitor in non-small-cell lung cancer. J. Cancer Res. Clin. 2012;138:2069–2077. doi: 10.1007/s00432-012-1291-2. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

