Characterization of New Oligosaccharides Obtained by An Enzymatic Cleavage of the Exopolysaccharide Produced by the Deep-Sea Bacterium Alteromonas infernus Using its Cell Extract
- PMID: 31546751
- PMCID: PMC6804119
- DOI: 10.3390/molecules24193441
Characterization of New Oligosaccharides Obtained by An Enzymatic Cleavage of the Exopolysaccharide Produced by the Deep-Sea Bacterium Alteromonas infernus Using its Cell Extract
Abstract
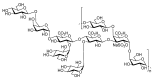
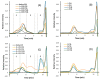
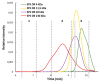
Bacteria from deep-sea hydrothermal vents constitute an attractive source of bioactive molecules. In particular, exopolysaccharides (EPS) produced by these bacteria become a renewable source of both biocompatible and biodegradable molecules. The low molecular weight (LMW) derivatives of the GY785 EPS produced by the deep-sea hydrothermal vent strain Alteromonas infernus have previously displayed some biological properties, similar to those of glycosaminoglycans (GAG), explored in cancer and tissue engineering. These GAG-mimetic derivatives are obtained through a free radical depolymerization process, which could, however, affect their structural integrity. In a previous study, we have shown that A. infernus produces depolymerizing enzymes active on its own EPS. In the present study, an enzymatic reaction was optimized to generate LMW derivatives of the GY785 EPS, which could advantageously replace the present bioactive derivatives obtained by a chemical process. Analysis by mass spectrometry of the oligosaccharide fractions released after enzymatic treatment revealed that mainly a lyase activity was responsible for the polysaccharide depolymerization. The repeating unit of the GY785 EPS produced by enzyme cleavage was then fully characterized.
Keywords: Alteromonas infernus; deep-sea bacterium; enzymatic depolymerization; exopolysaccharides; glycosaminoglycan-mimetic; mass spectrometry; structural analysis; wild-type strain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Held M.A., Jiang N., Basu D., Showalter A.M., Faik A. Plant Cell Wall Polysaccharides: Structure and Biosynthesis. Springer Science and Business Media LLC; Berlin, Germany: 2015. pp. 3–54.
-
- Vázquez J., Rodríguez-Amado I., Montemayor M., Fraguas J., González M., Murado M. Chondroitin Sulfate, Hyaluronic Acid and Chitin/Chitosan Production Using Marine Waste Sources: Characteristics, Applications and Eco-Friendly Processes: A Review. Mar. Drugs. 2013;11:747–774. doi: 10.3390/md11030747. - DOI - PMC - PubMed
-
- Öner E.T. Waste Energy for Life Cycle Assessment. Springer Science and Business Media LLC; Berlin, Germany: 2013. Microbial Production of Extracellular Polysaccharides from Biomass; pp. 35–56.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

