Heightened TLR7/9-Induced IL-10 and CXCL13 Production with Dysregulated NF-ҝB Activation in CD11chiCD11b+ Dendritic Cells in NZB/W F1 Mice
- PMID: 31546763
- PMCID: PMC6770860
- DOI: 10.3390/ijms20184639
Heightened TLR7/9-Induced IL-10 and CXCL13 Production with Dysregulated NF-ҝB Activation in CD11chiCD11b+ Dendritic Cells in NZB/W F1 Mice
Abstract
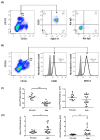
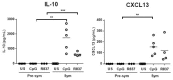
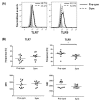
Systemic lupus erythematosus (SLE) is a chronic, multifactorial autoimmune disease that predominantly affects young females. Dysregulation of different immune cell populations leads to self-tolerance breakdown and subsequent multiple organ damage as the disease develops. Plasmacytoid dendritic cells (pDCs) are potent producers of type I interferon (IFN), while myeloid dendritic cells (mDCs) are more specialized in antigen presentations. We have previously reported that bone-marrow (BM)-derived pDCs from the murine lupus model New Zealand black/white F1 (BWF1) possess abnormalities. Therefore, this study continues to investigate what aberrant properties peripheral pDCs and mDCs possess in BWF1 and how they mediate SLE progression, by comparing their properties in pre-symptomatic and symptomatic mice. Results showed that CD11chiCD11b+ myeloid DCs expanded during the disease state with down-regulation of co-stimulatory molecules and major histocompatibility complex class II molecules (MHC II), but their capacity to stimulate T cells was not hampered. During the disease state, this subset of mDCs displayed heightened toll-like receptors 7 and 9 (TLR 7/9) responses with increased interleukin 10 (IL-10) and C-X-C motif chemokine ligand 13 (CXCL13) expressions. Moreover, the expressions of myeloid differentiation primary response 88 (Myd88) and nuclear factor kappa B subunit 1 (Nfkb1) were higher in CD11chiCD11b+ DCs at the disease stage, leading to higher nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) p65 phosphorylation activity. In summary, we reported aberrant phenotypic properties with enhanced TLR7/9 responses of CD11chiCD11b+ DCs in SLE mediated by aberrant NF-κB signaling pathway. Our findings add additional and novel information to our current understanding of the role of DCs in lupus immunopathogenesis. Lastly, molecular candidates in the NF-κB pathway should be exploited for developing therapeutic targets for SLE.
Keywords: C-X-C motif chemokine ligand 13; Toll-like receptor 7; Toll-like receptor 9; interleukin 10; myeloid dendritic cell; systemic lupus erythematosus.
Conflict of interest statement
The authors declare no conflict of interests.
Figures



References
-
- Petri M., Orbai A.M., Alarcon G.S., Gordon C., Merrill J.T., Fortin P.R., Bruce I.N., Isenberg D., Wallace D.J., Nived O., et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–2686. doi: 10.1002/art.34473. - DOI - PMC - PubMed
-
- Kumagai Y., Kumar H., Koyama S., Kawai T., Takeuchi O., Akira S. Cutting Edge: TLR-Dependent Viral Recognition Along with Type I IFN Positive Feedback Signaling Masks the Requirement of Viral Replication for IFN-α Production in Plasmacytoid Dendritic Cells. J. Immunol. 2009;182:3960–3964. doi: 10.4049/jimmunol.0804315. - DOI - PubMed
-
- Young L.J., Wilson N.S., Schnorrer P., Proietto A., ten Broeke T., Matsuki Y., Mount A.M., Belz G.T., O’Keeffe M., Ohmura-Hoshino M., et al. Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nat. Immunol. 2008;9:1244–1252. doi: 10.1038/ni.1665. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

