Expression of Renin-Angiotensin System Components in the Taste Organ of Mice
- PMID: 31546789
- PMCID: PMC6770651
- DOI: 10.3390/nu11092251
Expression of Renin-Angiotensin System Components in the Taste Organ of Mice
Abstract
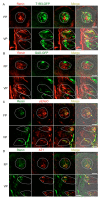
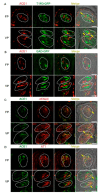
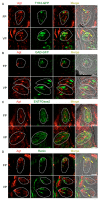
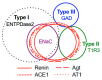
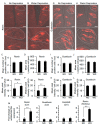
The systemic renin-angiotensin system (RAS) is an important regulator of body fluid and sodium homeostasis. Angiotensin II (AngII) is a key active product of the RAS. We previously revealed that circulating AngII suppresses amiloride-sensitive salt taste responses and enhances the responses to sweet compounds via the AngII type 1 receptor (AT1) expressed in taste cells. However, the molecular mechanisms underlying the modulation of taste function by AngII remain uncharacterized. Here we examined the expression of three RAS components, namely renin, angiotensinogen, and angiotensin-converting enzyme-1 (ACE1), in mouse taste tissues. We found that all three RAS components were present in the taste buds of fungiform and circumvallate papillae and co-expressed with αENaC (epithelial sodium channel α-subunit, a salt taste receptor) or T1R3 (taste receptor type 1 member 3, a sweet taste receptor component). Water-deprived mice exhibited significantly increased levels of renin expression in taste cells (p < 0.05). These results indicate the existence of a local RAS in the taste organ and suggest that taste function may be regulated by both locally-produced and circulating AngII. Such integrated modulation of peripheral taste sensitivity by AngII may play an important role in sodium/calorie homeostasis.
Keywords: angiotensin II; angiotensin-converting enzyme; angiotensinogen; renin; sodium taste; taste.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Braun-Menéndez E., Fasciolo J.C., Leloir L.F., Munoz J.M. La sustancia hipertensora de la sangre del riñón isquemido. Rev. Soc. Argent. Biol. 1939;15:420–425.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

