Denatonium Benzoate-Induces Oxidative Stress in the Heart and Kidney of Chinese Fast Yellow Chickens by Regulating Apoptosis, Autophagy, Antioxidative Activities and Bitter Taste Receptor Gene Expressions
- PMID: 31546822
- PMCID: PMC6770773
- DOI: 10.3390/ani9090701
Denatonium Benzoate-Induces Oxidative Stress in the Heart and Kidney of Chinese Fast Yellow Chickens by Regulating Apoptosis, Autophagy, Antioxidative Activities and Bitter Taste Receptor Gene Expressions
Abstract
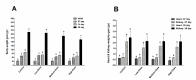
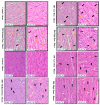
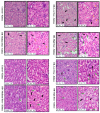
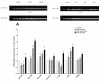
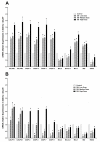
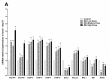
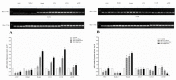
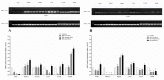
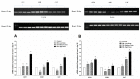
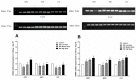
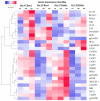
The sense of taste which tells us which prospective foods are nutritious, poisonous and harmful is essential for the life of the organisms. Denatonium benzoate (DB) is a bitter taste agonist known for its activation of bitter taste receptors in different cells. The aim of the current study was to investigate the mRNA expressions of bitter taste, downstream signaling effectors, apoptosis-, autophagy- and antioxidant-related genes and effector signaling pathways in the heart/kidney of chickens after DB dietary exposure. We randomly assigned 240, 1-day-old Chinese Fast Yellow chicks into four groups with five replicates of 12 chicks and studied them for 28 consecutive days. The dietary treatments consisted of basal diet and feed containing DB (5, 20 and 100 mg/kg). The results revealed that dietary DB impaired (p < 0.05) the growth performance of the chickens. Haemotoxylin and eosin staining and TUNEL assays confirmed that medium and high doses of DB damaged the epithelial cells of heart/kidney and induced apoptosis and autophagy. Remarkably, the results of RT-PCR and qRT-PCR indicated that different doses of DB gradually increased (p < 0.05) mRNA expressions of bitter taste, signaling effectors, apoptosis-, autophagy- and antioxidant- related genes on day 7 in a dose-response manner, while, these expressions were decreased (p < 0.05) subsequently by day-28 but exceptional higher (P < 0.05) expressions were observed in the high-dose DB groups of chickens. In conclusion, DB exerts adverse effects on the heart/kidney of chickens in a dose-response manner via damaging the epithelium of the heart/kidney by inducing apoptosis, autophagy associated with bitter taste and effector gene expressions. Correlation analyses for apoptosis/autophagy showed agonistic relationships. Our data provide a novel perspective for understanding the interaction of bitter taste, apoptosis, autophagy and antioxidative genes with bitter taste strong activators in the heart/kidney of chicken. These insights might help the feed industries and pave the way toward innovative directions in chicken husbandry.
Keywords: apoptosis; autophagy; bitter taste receptors; chicken; denatonium benzoate; heart; kidney.
Conflict of interest statement
The author declares no conflict of interest is present in this manuscript
Figures





References
-
- Boland M., Fordyce H., Greenwood R. Enzymes of nitrogen metabolism in legume nodules: A comparative study. Funct. Plant Biol. 1978;5:553–559. doi: 10.1071/PP9780553. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

