γ-H2AX Foci Persistence at Chromosome Break Suggests Slow and Faithful Repair Phases Restoring Chromosome Integrity
- PMID: 31546867
- PMCID: PMC6770925
- DOI: 10.3390/cancers11091397
γ-H2AX Foci Persistence at Chromosome Break Suggests Slow and Faithful Repair Phases Restoring Chromosome Integrity
Abstract
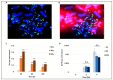
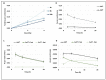
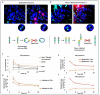
Many toxic agents can cause DNA double strand breaks (DSBs), which are in most cases quickly repaired by the cellular machinery. Using ionising radiation, we explored the kinetics of DNA lesion signaling and structural chromosome aberration formation at the intra- and inter-chromosomal level. Using a novel approach, the classic Premature Chromosome Condensation (PCC) was combined with γ-H2AX immunofluorescence staining in order to unravel the kinetics of DNA damage signalisation and chromosome repair. We identified an early mechanism of DNA DSB joining that occurs within the first three hours post-irradiation, when dicentric chromosomes and chromosome exchanges are formed. The slower and significant decrease of "deleted chromosomes" and 1 acentric telomere fragments observed until 24 h post-irradiation, leads to the conclusion that a second and error-free repair mechanism occurs. In parallel, we revealed remaining signalling of γ-H2AX foci at the site of chromosome fusion long after the chromosome rearrangement formation. Moreover there is important signalling of foci on the site of telomere and sub-telomere sequences suggesting either a different function of γ-H2AX signalling in these regions or an extreme sensibility of the telomere sequences to DNA damage that remains unrepaired 24 h post-irradiation. In conclusion, chromosome repair happens in two steps, including a last and hardly detectable one because of restoration of the chromosome integrity.
Keywords: DNA repair; chromosome; dicentric chromosome; irradiation; premature chromosome condensation; telomere; γ-H2AX.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

