Non-Enzymatic Electrochemical Sensor Based on Sliver Nanoparticle-Decorated Carbon Nanotubes
- PMID: 31546874
- PMCID: PMC6766966
- DOI: 10.3390/molecules24183411
Non-Enzymatic Electrochemical Sensor Based on Sliver Nanoparticle-Decorated Carbon Nanotubes
Abstract
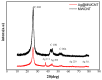
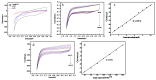
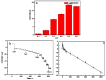
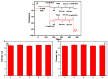
The authors report a non-enzymatic electrochemical sensor based on a sliver nanoparticle-decorated carbon nanotube (AgNPs-MWCNT). Highly-dispersed AgNPs were loaded on the MWCNT surface though a simple and facile two-step method. The morphology, components, and the size of the AgNPs-MWCNT nanocomposite were characterized by transmission electron microscopy, X-ray diffraction, and ICP analysis. Benefitting from the synergistic effect between the AgNPs and MWCNT, the AgNPs-MWCNT nanocomposite exhibited high electrocatalytic activity for H2O2; the AgNPs-MWCNT electrochemical sensor was prepared by coating the AgNPs-MWCNT nanocomposite on a glassy carbon electrode, and it showed a fast and sensitive response to H2O2 with a linear range of 1 to 1000 μM. The detection limit was 0.38 μM (S/N = 3). The sensor was applied to detect H2O2 in spiked human blood serum samples with satisfactory results.
Keywords: carbon nanotubes; hydrogen peroxide; nonenzymatic; sensor; silver nanoparticles.
Conflict of interest statement
The author(s) declare that they have no competing interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Varghese S.H., Nair R., Nair B.G., Hanajiri T., Maekawa T., Yoshida Y., Kumar S. Sensors based on carbon nanotubes and their applications: A review. Curr. Nanosci. 2010;6:331–346. doi: 10.2174/157341310791659053. - DOI
-
- Gooding J.J. Nanostructuring electrodes with carbon nanotubes: A review on electrochemistry and applications for sensing. Electrochim. Acta. 2005;50:3049–3060. doi: 10.1016/j.electacta.2004.08.052. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

