Advances in the Asymmetric Total Synthesis of Natural Products Using Chiral Secondary Amine Catalyzed Reactions of α,β-Unsaturated Aldehydes
- PMID: 31546876
- PMCID: PMC6767148
- DOI: 10.3390/molecules24183412
Advances in the Asymmetric Total Synthesis of Natural Products Using Chiral Secondary Amine Catalyzed Reactions of α,β-Unsaturated Aldehydes
Abstract
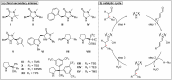
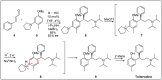
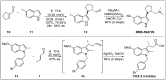
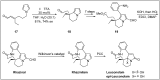
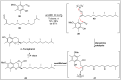
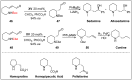
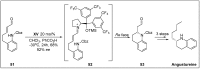
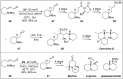
Chirality is one of the most important attributes for its presence in a vast majority of bioactive natural products and pharmaceuticals. Asymmetric organocatalysis methods have emerged as a powerful methodology for the construction of highly enantioenriched structural skeletons of the target molecules. Due to their extensive application of organocatalysis in the total synthesis of bioactive molecules and some of them have been used in the industrial synthesis of drugs have attracted increasing interests from chemists. Among the chiral organocatalysts, chiral secondary amines (MacMillan's catalyst and Jorgensen's catalyst) have been especially considered attractive strategies because of their impressive efficiency. Herein, we outline advances in the asymmetric total synthesis of natural products and relevant drugs by using the strategy of chiral secondary amine catalyzed reactions of α,β-unsaturated aldehydes in the last eighteen years.
Keywords: asymmetric total synthesis; bioactive natural products; chiral secondary amines; pharmaceuticals; α,β-unsaturated aldehydes.
Conflict of interest statement
The author declares no conflict of interest.
Figures











Similar articles
-
Asymmetric direct aldol reactions of acetoacetals catalyzed by a simple chiral primary amine.J Org Chem. 2009 Dec 18;74(24):9521-3. doi: 10.1021/jo9021259. J Org Chem. 2009. PMID: 19883088
-
Chiral Aldehyde Catalysis-Enabled Asymmetric α-Functionalization of Activated Primary Amines.Acc Chem Res. 2024 Mar 5;57(5):776-794. doi: 10.1021/acs.accounts.3c00804. Epub 2024 Feb 21. Acc Chem Res. 2024. PMID: 38381559
-
The diarylprolinol silyl ether system: a general organocatalyst.Acc Chem Res. 2012 Feb 21;45(2):248-64. doi: 10.1021/ar200149w. Epub 2011 Aug 17. Acc Chem Res. 2012. PMID: 21848275
-
Asymmetric Organocatalysis: A Survival Guide to Medicinal Chemists.Molecules. 2022 Dec 29;28(1):271. doi: 10.3390/molecules28010271. Molecules. 2022. PMID: 36615465 Free PMC article. Review.
-
Asymmetric organocatalysis: an enabling technology for medicinal chemistry.Chem Soc Rev. 2021 Feb 15;50(3):1522-1586. doi: 10.1039/d0cs00196a. Chem Soc Rev. 2021. PMID: 33496291 Review.
Cited by
-
Asymmetric Synthesis of US-FDA Approved Drugs over Five Years (2016-2020): A Recapitulation of Chirality.Pharmaceuticals (Basel). 2023 Feb 22;16(3):339. doi: 10.3390/ph16030339. Pharmaceuticals (Basel). 2023. PMID: 36986439 Free PMC article. Review.
-
Origin of rate enhancement and asynchronicity in iminium catalyzed Diels-Alder reactions.Chem Sci. 2020 Jul 9;11(31):8105-8112. doi: 10.1039/d0sc02901g. Chem Sci. 2020. PMID: 34094173 Free PMC article.
-
Design and Synthesis of Helical N-Terminal L-Prolyl Oligopeptides Possessing Hydrocarbon Stapling.Molecules. 2020 Oct 13;25(20):4667. doi: 10.3390/molecules25204667. Molecules. 2020. PMID: 33066194 Free PMC article.
-
Bioactive natural products in COVID-19 therapy.Front Pharmacol. 2022 Aug 19;13:926507. doi: 10.3389/fphar.2022.926507. eCollection 2022. Front Pharmacol. 2022. PMID: 36059994 Free PMC article. Review.
-
Editorial to the Special Issue "Total Synthesis of Natural Products: A Themed Issue Dedicated to Professor Dr. Dieter Schinzer for His 65th Birthday".Molecules. 2020 Dec 10;25(24):5841. doi: 10.3390/molecules25245841. Molecules. 2020. PMID: 33322027 Free PMC article.
References
-
- Ahrendt K.A., Borths C.J., MacMillan D.W. New strategies for organic catalysis: The first highly enantioselective organocatalytic diels—alder reaction. J. Am. Chem. Soc. 2000;122:4243–4244. doi: 10.1021/ja000092s. - DOI
-
- Franzén J., Marigo M., Fielenbach D., Wabnitz T.C., Kjærsgaard A., Jørgensen K.A. A general organocatalyst for direct α-functionalization of aldehydes: Stereoselective C−C, C−N, C−F, C−Br, and C−S bond-forming reactions. scope and mechanistic insights. J. Am. Chem. Soc. 2005;127:18296–18304. doi: 10.1021/ja056120u. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

