Functions and Regulatory Mechanisms of lncRNAs in Skeletal Myogenesis, Muscle Disease and Meat Production
- PMID: 31546877
- PMCID: PMC6769631
- DOI: 10.3390/cells8091107
Functions and Regulatory Mechanisms of lncRNAs in Skeletal Myogenesis, Muscle Disease and Meat Production
Abstract
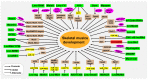
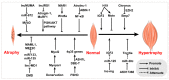
Myogenesis is a complex biological process, and understanding the regulatory network of skeletal myogenesis will contribute to the treatment of human muscle related diseases and improvement of agricultural animal meat production. Long noncoding RNAs (lncRNAs) serve as regulators in gene expression networks, and participate in various biological processes. Recent studies have identified functional lncRNAs involved in skeletal muscle development and disease. These lncRNAs regulate the proliferation, differentiation, and fusion of myoblasts through multiple mechanisms, such as chromatin modification, transcription regulation, and microRNA sponge activity. In this review, we presented the latest advances regarding the functions and regulatory activities of lncRNAs involved in muscle development, muscle disease, and meat production. Moreover, challenges and future perspectives related to the identification of functional lncRNAs were also discussed.
Keywords: lncRNA; meat production; muscle disease; myogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical