Dynamic Interplay between Pericytes and Endothelial Cells during Sprouting Angiogenesis
- PMID: 31546913
- PMCID: PMC6770602
- DOI: 10.3390/cells8091109
Dynamic Interplay between Pericytes and Endothelial Cells during Sprouting Angiogenesis
Abstract
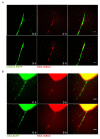
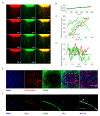
Vascular physiology relies on the concerted dynamics of several cell types, including pericytes, endothelial, and vascular smooth muscle cells. The interactions between such cell types are inherently dynamic and are not easily described with static, fixed, experimental approaches. Pericytes are mural cells that support vascular development, remodeling, and homeostasis, and are involved in a number of pathological situations including cancer. The dynamic interplay between pericytes and endothelial cells is at the basis of vascular physiology and few experimental tools exist to properly describe and study it. Here we employ a previously developed ex vivo murine aortic explant to study the formation of new blood capillary-like structures close to physiological situation. We develop several mouse models to culture, identify, characterize, and follow simultaneously single endothelial cells and pericytes during angiogenesis. We employ microscopy and image analysis to dissect the interactions between cell types and the process of cellular recruitment on the newly forming vessel. We find that pericytes are recruited on the developing sprout by proliferation, migrate independently from endothelial cells, and can proliferate on the growing capillary. Our results help elucidating several relevant mechanisms of interactions between endothelial cells and pericytes.
Keywords: NG2; aortic ring assay; cancer; endothelial cells; pericytes; sprouting angiogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures