Injectable Hydrogels for Cancer Therapy over the Last Decade
- PMID: 31546921
- PMCID: PMC6781516
- DOI: 10.3390/pharmaceutics11090486
Injectable Hydrogels for Cancer Therapy over the Last Decade
Abstract
The interest in injectable hydrogels for cancer treatment has been significantly growing over the last decade, due to the availability of a wide range of starting polymer structures with tailored features and high chemical versatility. Many research groups are working on the development of highly engineered injectable delivery vehicle systems suitable for combined chemo-and radio-therapy, as well as thermal and photo-thermal ablation, with the aim of finding out effective solutions to overcome the current obstacles of conventional therapeutic protocols. Within this work, we have reviewed and discussed the most recent injectable hydrogel systems, focusing on the structure and properties of the starting polymers, which are mainly classified into natural or synthetic sources. Moreover, mapping the research landscape of the fabrication strategies, the main outcome of each system is discussed in light of possible clinical applications.
Keywords: anticancer activity; drug delivery; injectable hydrogels; natural polymers; stimuli-responsive materials; synthetic polymers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

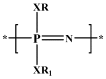
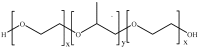


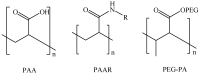




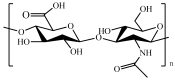



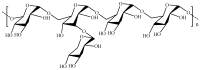


References
-
- Ko D.Y., Shinde U.P., Yeon B., Jeong B. Recent progress of in situ formed gels for biomedical applications. Prog. Polym. Sci. 2013;38:672–701. doi: 10.1016/j.progpolymsci.2012.08.002. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

