Pharmacokinetic Properties of Fluorescently Labelled Hydroxypropyl-Beta-Cyclodextrin
- PMID: 31546989
- PMCID: PMC6843445
- DOI: 10.3390/biom9100509
Pharmacokinetic Properties of Fluorescently Labelled Hydroxypropyl-Beta-Cyclodextrin
Abstract
2-Hydroxypropyl-beta-cyclodextrin (HPBCD) is utilized in the formulation of pharmaceutical products and recently orphan designation was granted for the treatment of Niemann-Pick disease, type C. The exact mechanism of HPBCD action and side effects are not completely explained. We used fluorescently labelled hydroxypropyl-beta-cyclodextrin (FITC-HPBCD) to study its pharmacokinetic parameters in mice and compare with native HPBCD data. We found that FITC-HPBCD has fast distribution and elimination, similar to HPBCD. Interestingly animals could be divided into two groups, where the pharmacokinetic parameters followed or did not follow the two-compartment, first-order kinetic model. Tissue distribution studies revealed, that a significant amount of FITC-HPBCD could be detected in kidneys after 60 min treatment, due to its renal excretion. Ex vivo fluorescent imaging showed that fluorescence could be measured in lung, liver, brain and spleen after 30 min of treatment. To model the interaction and cellular distribution of FITC-HPBCD in the wall of blood vessels, we treated human umbilical vein endothelial cells (HUVECs) with FITC-HPBCD and demonstrated for the first time that this compound could be detected in the cytoplasm in small vesicles after 30 min of treatment. FITC-HPBCD has similar pharmacokinetic to HPBCD and can provide new information to the detailed mechanism of action of HPBCD.
Keywords: HUVECs; endocytosis; fluorescence; hydroxypropyl-beta-cyclodextrin; pharmacokinetics.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



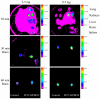

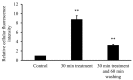
References
-
- Committee for Orphan Medicinal Products, European Medicines Agency Public Summary of Opinion on Orphan Designation. EMA/COMP/546608/2011 Rev.1. [(accessed on 18 March 2018)];2015 Mar 12; Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Orphan_designation/....
-
- Ory D.S., Ottinger E.A., Farhat N.Y., King K.A., Jiang X., Weissfeld L., Berry-Kravis E., Davidson C.D., Bianconi S., Keener L.A., et al. Intrathecal 2-hydroxypropyl-beta-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: A non-randomised, open-label, phase 1–2 trial. Lancet. 2017;390:1758–1768. doi: 10.1016/S0140-6736(17)31465-4. - DOI - PMC - PubMed
-
- FDA List of Inactive Pharmaceutical Ingredients. [(accessed on 18 March 2018)]; Available online: https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm?event=browseBy....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

