Brain-Immune Alterations and Mitochondrial Dysfunctions in a Mouse Model of Paediatric Autoimmune Disorder Associated with Streptococcus: Exacerbation by Chronic Psychosocial Stress
- PMID: 31547098
- PMCID: PMC6833026
- DOI: 10.3390/jcm8101514
Brain-Immune Alterations and Mitochondrial Dysfunctions in a Mouse Model of Paediatric Autoimmune Disorder Associated with Streptococcus: Exacerbation by Chronic Psychosocial Stress
Abstract
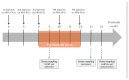
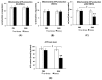
Adverse psychosocial experiences have been shown to modulate individual responses to immune challenges and affect mitochondrial functions. The aim of this study was to investigate inflammation and immune responses as well as mitochondrial bioenergetics in an experimental model of Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcus (PANDAS). Starting in adolescence (postnatal day 28), male SJL/J mice were exposed to five injections (interspaced by two weeks) with Group-A beta-haemolytic streptococcus (GAS) homogenate. Mice were exposed to chronic psychosocial stress, in the form of protracted visual exposure to an aggressive conspecific, for four weeks. Our results indicate that psychosocial stress exacerbated individual response to GAS administrations whereby mice exposed to both treatments exhibited altered cytokine and immune-related enzyme expression in the hippocampus and hypothalamus. Additionally, they showed impaired mitochondrial respiratory chain complexes IV and V, and reduced adenosine triphosphate (ATP) production by mitochondria and ATP content. These brain abnormalities, observed in GAS-Stress mice, were associated with blunted titers of plasma corticosterone. Present data support the hypothesis that challenging environmental conditions, in terms of chronic psychosocial stress, may exacerbate the long-term consequences of exposure to GAS processes through the promotion of central immunomodulatory and oxidative stress.
Keywords: Adverse emotional experience; Animal Models; Immunity; Mitochondrial bioenergetics; Neuroinflammation; PANDAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Köhler-Forsberg O., Petersen L., Gasse C., Mortensen P.B., Dalsgaard S., Yolken R.H., Mors O., Benros M.E. A Nationwide Study in Denmark of the Association between Treated Infections and the Subsequent Risk of Treated Mental Disorders in Children and Adolescents. JAMA Psychiatry. 2018 doi: 10.1001/jamapsychiatry.2018.3428. - DOI - PMC - PubMed
-
- Tylee D.S., Sun J., Hess J.L., Tahir M.A., Sharma E., Malik R., Worrall B.B., Levine A.J., Martinson J.J., Nejentsev S., et al. Genetic correlations among psychiatric and immune-related phenotypes based on genome-wide association data. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2018;177:641–657. doi: 10.1002/ajmg.b.32652. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

