Increased ROS Scavenging and Antioxidant Efficiency of Chlorogenic Acid Compound Delivered via a Chitosan Nanoparticulate System for Efficient In Vitro Visualization and Accumulation in Human Renal Adenocarcinoma Cells
- PMID: 31547100
- PMCID: PMC6801874
- DOI: 10.3390/ijms20194667
Increased ROS Scavenging and Antioxidant Efficiency of Chlorogenic Acid Compound Delivered via a Chitosan Nanoparticulate System for Efficient In Vitro Visualization and Accumulation in Human Renal Adenocarcinoma Cells
Abstract
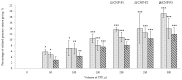
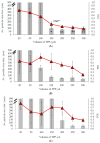
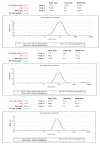
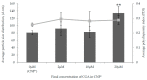
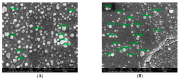
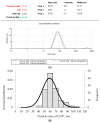
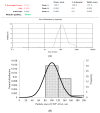
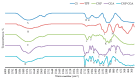
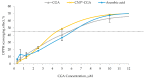
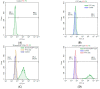
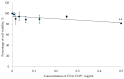
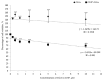
Naturally existing Chlorogenic acid (CGA) is an antioxidant-rich compound reported to act a chemopreventive agent by scavenging free radicals and suppressing cancer-causing mechanisms. Conversely, the compound's poor thermal and pH (neutral and basic) stability, poor solubility, and low cellular permeability have been a huge hindrance for it to exhibit its efficacy as a nutraceutical compound. Supposedly, encapsulation of CGA in chitosan nanoparticles (CNP), nano-sized colloidal delivery vector, could possibly assist in enhancing its antioxidant properties, in vitro cellular accumulation, and increase chemopreventive efficacy at a lower concentration. Hence, in this study, a stable, monodispersed, non-toxic CNP synthesized via ionic gelation method at an optimum parameter (600 µL of 0.5 mg/mL of chitosan and 200 µL of 0.7 mg/mL of tripolyphosphate), denoted as CNP°, was used to encapsulate CGA. Sequence of physicochemical analyses and morphological studies were performed to discern the successful formation of the CNP°-CGA hybrid. Antioxidant property (studied via DPPH (1,1-diphenyl-2-picrylhydrazyl) assay), in vitro antiproliferative activity of CNP°-CGA, and in vitro accumulation of fluorescently labeled (FITC) CNP°-CGA in cancer cells were evaluated. Findings revealed that successful formation of CNP°-CGA hybrid was reveled through an increase in particle size 134.44 ± 18.29 nm (polydispersity index (PDI) 0.29 ± 0.03) as compared to empty CNP°, 80.89 ± 5.16 nm (PDI 0.26 ± 0.01) with a maximal of 12.04 μM CGA loaded per unit weight of CNP° using 20 µM of CGA. This result correlated with Fourier-Transform Infrared (FTIR) spectroscopic analysis, transmission Electron Microscopy (TEM) and field emission scanning (FESEM) electron microscopy, and ImageJ evaluation. The scavenging activity of CNP°-CGA (IC50 5.2 ± 0.10 µM) were conserved and slightly higher than CNP° (IC50 6.4±0.78 µM). An enhanced cellular accumulation of fluorescently labeled CNP°-CGA in the human renal cancer cells (786-O) as early as 30 min and increased time-dependently were observed through fluorescent microscopic visualization and flow cytometric assessment. A significant concentration-dependent antiproliferation activity of encapsulated CGA was achieved at IC50 of 16.20 µM as compared to CGA itself (unable to determine from the cell proliferative assay), implying that the competent delivery vector, chitosan nanoparticle, is able to enhance the intracellular accumulation, antiproliferative activity, and antioxidant properties of CGA at lower concentration as compared to CGA alone.
Keywords: 1,1-Diphenyl-2-picrylhydrazine assay; MTT assay; chemopreventive; chitosan nanoparticles; chlorogenic acid; drug encapsulated chitosan nanoparticles; in vitro accumulation of encapsulated chlorogenic acid; nanobiotechnology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
In vitro cellular localization and efficient accumulation of fluorescently tagged biomaterials from monodispersed chitosan nanoparticles for elucidation of controlled release pathways for drug delivery systems.Int J Nanomedicine. 2018 Sep 5;13:5075-5095. doi: 10.2147/IJN.S164843. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30233174 Free PMC article.
-
Improved Chemical Stability and Antiproliferative Activities of Curcumin-Loaded Nanoparticles with a Chitosan Chlorogenic Acid Conjugate.J Agric Food Chem. 2017 Dec 13;65(49):10812-10819. doi: 10.1021/acs.jafc.7b04451. Epub 2017 Nov 29. J Agric Food Chem. 2017. PMID: 29155582
-
Increased Loading, Efficacy and Sustained Release of Silibinin, a Poorly Soluble Drug Using Hydrophobically-Modified Chitosan Nanoparticles for Enhanced Delivery of Anticancer Drug Delivery Systems.Nanomaterials (Basel). 2017 Nov 8;7(11):379. doi: 10.3390/nano7110379. Nanomaterials (Basel). 2017. PMID: 29117121 Free PMC article.
-
Biomedical and Pharmacological Uses of Fluorescein Isothiocyanate Chitosan-Based Nanocarriers.Macromol Biosci. 2021 Jan;21(1):e2000312. doi: 10.1002/mabi.202000312. Epub 2020 Oct 4. Macromol Biosci. 2021. PMID: 33016007 Review.
-
Recent Updates on the Therapeutics Benefits, Clinical Trials, and Novel Delivery Systems of Chlorogenic Acid for the Management of Diseases with a Special Emphasis on Ulcerative Colitis.Curr Pharm Des. 2024;30(6):420-439. doi: 10.2174/0113816128295753240129074035. Curr Pharm Des. 2024. PMID: 38299405 Review.
Cited by
-
Investigation of Bioactive Complexes of Chitosan and Green Coffee Bean or Artichoke Extracts.Molecules. 2023 Jul 12;28(14):5356. doi: 10.3390/molecules28145356. Molecules. 2023. PMID: 37513230 Free PMC article.
-
Cinnamic Acid Derivatives as Potential Multifunctional Agents in Cosmetic Formulations Used for Supporting the Treatment of Selected Dermatoses.Molecules. 2024 Dec 9;29(23):5806. doi: 10.3390/molecules29235806. Molecules. 2024. PMID: 39683963 Free PMC article. Review.
-
Pharmacologic Overview of Chlorogenic Acid and its Metabolites in Chronic Pain and Inflammation.Curr Neuropharmacol. 2020;18(3):216-228. doi: 10.2174/1570159X17666191021111809. Curr Neuropharmacol. 2020. PMID: 31631820 Free PMC article. Review.
-
A Chinese Herbal Formula Suppresses Colorectal Cancer Migration and Vasculogenic Mimicry Through ROS/HIF-1α/MMP2 Pathway in Hypoxic Microenvironment.Front Pharmacol. 2020 May 15;11:705. doi: 10.3389/fphar.2020.00705. eCollection 2020. Front Pharmacol. 2020. PMID: 32499699 Free PMC article.
-
Hesperidin and Chlorogenic Acid Synergistically Inhibit the Growth of Breast Cancer Cells via Estrogen Receptor/Mitochondrial Pathway.Life (Basel). 2021 Sep 10;11(9):950. doi: 10.3390/life11090950. Life (Basel). 2021. PMID: 34575098 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

