Bacterial Cellulose: Production, Modification and Perspectives in Biomedical Applications
- PMID: 31547134
- PMCID: PMC6835293
- DOI: 10.3390/nano9101352
Bacterial Cellulose: Production, Modification and Perspectives in Biomedical Applications
Abstract
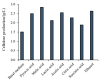
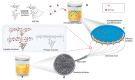
Bacterial cellulose (BC) is ultrafine, nanofibrillar material with an exclusive combination of properties such as high crystallinity (84%-89%) and polymerization degree, high surface area (high aspect ratio of fibers with diameter 20-100 nm), high flexibility and tensile strength (Young modulus of 15-18 GPa), high water-holding capacity (over 100 times of its own weight), etc. Due to high purity, i.e., absence of lignin and hemicellulose, BC is considered as a non-cytotoxic, non-genotoxic and highly biocompatible material, attracting interest in diverse areas with hallmarks in medicine. The presented review summarizes the microbial aspects of BC production (bacterial strains, carbon sources and media) and versatile in situ and ex situ methods applied in BC modification, especially towards bionic design for applications in regenerative medicine, from wound healing and artificial skin, blood vessels, coverings in nerve surgery, dura mater prosthesis, arterial stent coating, cartilage and bone repair implants, etc. The paper concludes with challenges and perspectives in light of further translation in highly valuable medical products.
Keywords: bacterial cellulose; biomedical applications; carbon source; ex situ modification; in situ modification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Wang S.S., Han Y.H., Chen J.L., Zhang D.C., Shi X.X., Ye Y.X., Chen D.L., Li M. Insights into bacterial cellulose biosynthesis from different carbon sources and the associated biochemical transformation pathways in Komagataeibacter sp. W1. Polymers. 2018;9:963. doi: 10.3390/polym10090963. - DOI - PMC - PubMed
-
- Trček J., Barja F. Updates on quick identification of acetic acid bacteria with a focus on the 16S–23S rRNA gene internal transcribed spacer and the analysis of cell proteins by MALDI-TOF mass spectrometry. Int. J. Food Microbiol. 2015;196:137–144. doi: 10.1016/j.ijfoodmicro.2014.12.003. - DOI - PubMed
-
- Škraban J., Cleenwerck I., Vandamme P., Fanedl L., Trček J. Genome sequences and description of novel exopolysaccharides producing species Komagataeibacter pomaceti sp. nov. and reclassification of Komagataeibacter kombuchae (Dutta and Gachhui 2007) Yamada et al. 2013 as a later heterotypic synonym of Komagataeibacter. Syst. Appl. Microbiol. 2018;41:581–592. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

