Cx43-Gap Junctions Accumulate at the Cytotoxic Immunological Synapse Enabling Cytotoxic T Lymphocyte Melanoma Cell Killing
- PMID: 31547237
- PMCID: PMC6769613
- DOI: 10.3390/ijms20184509
Cx43-Gap Junctions Accumulate at the Cytotoxic Immunological Synapse Enabling Cytotoxic T Lymphocyte Melanoma Cell Killing
Abstract
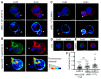
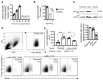
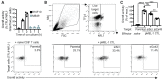
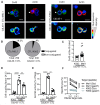
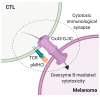
Upon tumor antigen recognition, cytotoxic T lymphocytes (CTLs) and target cells form specialized supramolecular structures, called cytotoxic immunological synapses, which are required for polarized delivery of cytotoxic granules. In previous reports, we described the accumulation of connexin 43 (Cx43)-formed gap junctions (GJs) at natural killer (NK) cell-tumor cell cytotoxic immunological synapse. In this report, we demonstrate the functional role of Cx43-GJs at the cytotoxic immunological synapse established between CTLs and melanoma cells during cytotoxicity. Using confocal microscopy, we evaluated Cx43 polarization to the contact site between CTLs isolated from pMEL-1 mice and B16F10 melanoma cells. We knocked down Cx43 expression in B16F10 cells and evaluated its role in the formation of functional GJs and the cytotoxic activity of CTLs, by calcein transfer and granzyme B activity assays, respectively. We found that Cx43 localizes at CTL/B16F10 intercellular contact sites via an antigen-dependent process. We also found that pMEL-1 CTLs but not wild-type naïve CD8+ T cells established functional GJs with B16F10 cells. Interestingly, we observed that Cx43-GJs were required for an efficient granzyme B activity in target B16F10 cells. Using an HLA-A2-restricted/MART-1-specific CD8+ T-cell clone, we confirmed these observations in human cells. Our results suggest that Cx43-channels are relevant components of cytotoxic immunological synapses and potentiate CTL-mediated tumor cell killing.
Keywords: connexin 43; cytotoxic T lymphocyte; cytotoxic immunological synapse; gap junctions; melanoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Regulation of gap junctions in melanoma and their impact on Melan-A/MART-1-specific CD8⁺ T lymphocyte emergence.J Mol Med (Berl). 2013 Oct;91(10):1207-20. doi: 10.1007/s00109-013-1058-5. Epub 2013 Jun 7. J Mol Med (Berl). 2013. PMID: 23744108
-
Gap junction intercellular communications regulate NK cell activation and modulate NK cytotoxic capacity.J Immunol. 2014 Feb 1;192(3):1313-9. doi: 10.4049/jimmunol.1301297. Epub 2013 Dec 27. J Immunol. 2014. PMID: 24376266
-
Flow Cytometry Evaluation of Gap Junction-Mediated Intercellular Communication Between Cytotoxic T Cells and Target Tumor Cells.Methods Mol Biol. 2021;2346:225-236. doi: 10.1007/7651_2020_326. Methods Mol Biol. 2021. PMID: 33029747
-
Connexin-Mediated Signaling at the Immunological Synapse.Int J Mol Sci. 2020 May 25;21(10):3736. doi: 10.3390/ijms21103736. Int J Mol Sci. 2020. PMID: 32466338 Free PMC article. Review.
-
The secretory synapse: the secrets of a serial killer.Immunol Rev. 2002 Nov;189:152-60. doi: 10.1034/j.1600-065x.2002.18913.x. Immunol Rev. 2002. PMID: 12445272 Review.
Cited by
-
Cell Adhesion Molecules in Plasticity and Metastasis.Mol Cancer Res. 2021 Jan;19(1):25-37. doi: 10.1158/1541-7786.MCR-20-0595. Epub 2020 Oct 1. Mol Cancer Res. 2021. PMID: 33004622 Free PMC article. Review.
-
Coordination of innate immune responses by connexins.Front Immunol. 2025 May 22;16:1594015. doi: 10.3389/fimmu.2025.1594015. eCollection 2025. Front Immunol. 2025. PMID: 40475783 Free PMC article. Review.
-
BCR-ABL1-driven exosome-miR130b-3p-mediated gap-junction Cx43 MSC intercellular communications imply therapies of leukemic subclonal evolution.Theranostics. 2023 Jul 3;13(12):3943-3963. doi: 10.7150/thno.83178. eCollection 2023. Theranostics. 2023. PMID: 37554265 Free PMC article.
-
Diversity of Intercellular Communication Modes: A Cancer Biology Perspective.Cells. 2024 Mar 12;13(6):495. doi: 10.3390/cells13060495. Cells. 2024. PMID: 38534339 Free PMC article. Review.
-
Regulation of autophagy fires up the cold tumor microenvironment to improve cancer immunotherapy.Front Immunol. 2022 Oct 10;13:1018903. doi: 10.3389/fimmu.2022.1018903. eCollection 2022. Front Immunol. 2022. PMID: 36300110 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

