Theoretical Studies of IR and NMR Spectral Changes Induced by Sigma-Hole Hydrogen, Halogen, Chalcogen, Pnicogen, and Tetrel Bonds in a Model Protein Environment
- PMID: 31547416
- PMCID: PMC6767630
- DOI: 10.3390/molecules24183329
Theoretical Studies of IR and NMR Spectral Changes Induced by Sigma-Hole Hydrogen, Halogen, Chalcogen, Pnicogen, and Tetrel Bonds in a Model Protein Environment
Abstract
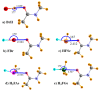
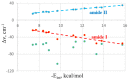
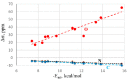
Various types of σ-hole bond complexes were formed with FX, HFY, H2FZ, and H3FT (X = Cl, Br, I; Y = S, Se, Te; Z = P, As, Sb; T = Si, Ge, Sn) as Lewis acid. In order to examine their interactions with a protein, N-methylacetamide (NMA), a model of the peptide linkage was used as the base. These noncovalent bonds were compared by computational means with H-bonds formed by NMA with XH molecules (X = F, Cl, Br, I). In all cases, the A-F bond, which lies opposite the base and is responsible for the σ-hole on the A atom (A refers to the bridging atom), elongates and its stretching frequency undergoes a shift to the red with a band intensification, much as what occurs for the X-H bond in a H-bond (HB). Unlike the NMR shielding decrease seen in the bridging proton of a H-bond, the shielding of the bridging A atom is increased. The spectroscopic changes within NMA are similar for H-bonds and the other noncovalent bonds. The C=O bond of the amide is lengthened and its stretching frequency red-shifted and intensified. The amide II band shifts to higher frequency and undergoes a small band weakening. The NMR shielding of the O atom directly involved in the bond rises, whereas the C and N atoms both undergo a shielding decrease. The frequency shifts of the amide I and II bands of the base as well as the shielding changes of the three pertinent NMA atoms correlate well with the strength of the noncovalent bond.
Keywords: NBO; NMR shielding; atomic charge; energy decomposition; stretching frequency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
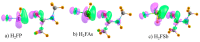
Similar articles
-
Effects of Halogen, Chalcogen, Pnicogen, and Tetrel Bonds on IR and NMR Spectra.Molecules. 2019 Aug 2;24(15):2822. doi: 10.3390/molecules24152822. Molecules. 2019. PMID: 31382402 Free PMC article.
-
Comparative Strengths of Tetrel, Pnicogen, Chalcogen, and Halogen Bonds and Contributing Factors.Molecules. 2018 Jul 10;23(7):1681. doi: 10.3390/molecules23071681. Molecules. 2018. PMID: 29996528 Free PMC article.
-
Chalcogen- and halogen-bonds involving SX2 (X = F, Cl, and Br) with formaldehyde.J Mol Model. 2016 Jul;22(7):167. doi: 10.1007/s00894-016-3037-6. Epub 2016 Jun 24. J Mol Model. 2016. PMID: 27342252
-
Hydrogen Bond and Other Lewis Acid-Lewis Base Interactions as Preliminary Stages of Chemical Reactions.Molecules. 2020 Oct 13;25(20):4668. doi: 10.3390/molecules25204668. Molecules. 2020. PMID: 33066201 Free PMC article. Review.
-
Non-classical Non-covalent σ-Hole Interactions in Protein Structure and Function: Concepts for Potential Protein Engineering Applications.Chem Asian J. 2023 Apr 3;18(7):e202300026. doi: 10.1002/asia.202300026. Epub 2023 Feb 24. Chem Asian J. 2023. PMID: 36764929 Review.
Cited by
-
Self-assembly of reversed bilayer vesicles through pnictogen bonding: water-stable supramolecular nanocontainers for organic solvents.Chem Sci. 2020 Apr 9;11(17):4374-4380. doi: 10.1039/d0sc00206b. eCollection 2020 May 7. Chem Sci. 2020. PMID: 33224458 Free PMC article.
-
Role of Non-Covalent Interactions in Carbonic Anhydrase I-Topiramate Complex Based on QM/MM Approach.Pharmaceuticals (Basel). 2023 Mar 23;16(4):479. doi: 10.3390/ph16040479. Pharmaceuticals (Basel). 2023. PMID: 37111236 Free PMC article.
-
Noncovalent Bonds through Sigma and Pi-Hole Located on the Same Molecule. Guiding Principles and Comparisons.Molecules. 2021 Mar 20;26(6):1740. doi: 10.3390/molecules26061740. Molecules. 2021. PMID: 33804617 Free PMC article. Review.
-
Chalcogen Bond as a Factor Stabilizing Ligand Conformation in the Binding Pocket of Carbonic Anhydrase IX Receptor Mimic.Int J Mol Sci. 2022 Nov 8;23(22):13701. doi: 10.3390/ijms232213701. Int J Mol Sci. 2022. PMID: 36430173 Free PMC article.
-
Crystal Structure Survey and Theoretical Analysis of Bifurcated Halogen Bonds.Cryst Growth Des. 2022 Nov 2;22(11):6521-6530. doi: 10.1021/acs.cgd.2c00726. Epub 2022 Oct 10. Cryst Growth Des. 2022. PMID: 36345386 Free PMC article.
References
-
- Hadzi D., Bratos S. Vibrational Spectroscopy of the hydrogen bond. In: Schuster P., Zundel G., Sandorfy C., editors. The Hydrogen Bond. Recent Developments in Theory and Experiments. Volume 2. North-Holland Publishing Co.; Amsterdam, The Netherlands: 1976. pp. 565–611.
-
- Gilli G., Gilli P. The Nature of the Hydrogen Bond. Oxford University Press; Oxford, UK: 2009. p. 313.
-
- Scheiner S. Hydrogen Bonding: A Theoretical Perspective. Oxford University Press; New York, NY, USA: 1997. p. 375.
-
- Rivera-Rivera L.A., McElmurry B.A., Scott K.W., Lucchese R.R., Bevan J.W. The Badger–Bauer Rule Revisited: Correlation of Proper Blue Frequency Shifts in the OC Hydrogen Acceptor with Morphed Hydrogen Bond Dissociation Energies in OC–HX (X = F, Cl, Br, I, CN, CCH) J. Phys. Chem. A. 2013;117:8477–8483. doi: 10.1021/jp4058516. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

