Induction of Urokinase Activity by Retinoic Acid in Two Cell Lines of Neuronal Origin
- PMID: 31547462
- PMCID: PMC6784121
- DOI: 10.3390/biomedicines7030070
Induction of Urokinase Activity by Retinoic Acid in Two Cell Lines of Neuronal Origin
Abstract
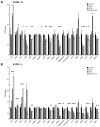
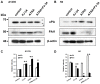
Retinoic acid is one of the most well-known agents able to induce differentiation in several types of tumours. Unfortunately, most of the tumours are refractive to the differentiation cues. The aim of this investigation was to analyse the effects of prolonged treatment with retinoic acid on two cell lines of neural origin refractive to differentiation. Cells were also treated with retinoic acid in combination with a poly(ADP-ribosyl) polymerase (PARP) inhibitor because PARP1 is a known chromatin modulator and can influence the process of differentiation. The main methods comprised tumour cell line culturing and treatment; analysis of RNA and protein expression after cell treatment; as well as analysis of urokinase activity, migration, and proliferation. Both cell lines continued to proliferate under the prolonged treatment and showed increase in urokinase plasminogen activator activity. Analysis of gene expression and cell phenotype revealed different mechanisms, which only in neuroblastoma H4 cells could indicate the process of epithelial-mesenchymal transition. The data collected indicate that the activity of the urokinase plasminogen activator, although belonging to an extracellular protease, does not necessary lead to epithelial-mesenchymal reprogramming and increase in cell migration but can have different outcomes depending on the intracellular milieu.
Keywords: PARP1; epithelial-mesenchymal transition; glioblastoma; neuroglioblastoma cell line; retinoic acid; urokinase.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






Similar articles
-
Retinoic acid upregulates the plasminogen activator system in human epidermal keratinocytes.J Invest Dermatol. 2001 May;116(5):778-84. doi: 10.1046/j.1523-1747.2001.01310.x. J Invest Dermatol. 2001. PMID: 11348470
-
Modulation of urokinase plasminogen activator system by poly(ADP-ribose)polymerase-1 inhibition.Cytotechnology. 2016 Aug;68(4):783-94. doi: 10.1007/s10616-014-9829-6. Epub 2014 Dec 4. Cytotechnology. 2016. PMID: 25471275 Free PMC article.
-
TGF-β2 promotes RPE cell invasion into a collagen gel by mediating urokinase-type plasminogen activator (uPA) expression.Exp Eye Res. 2013 Oct;115:13-21. doi: 10.1016/j.exer.2013.06.020. Epub 2013 Jun 28. Exp Eye Res. 2013. PMID: 23810810
-
Poly(ADP-ribosyl)ation, a molecular switch of transcription, shows an attractive relationship with urokinase expression.Thromb Haemost. 2005 Feb;93(2):220-7. doi: 10.1160/TH04-09-0605. Thromb Haemost. 2005. PMID: 15711736 Review.
-
[The urokinase-type plasminogen activator system and its role in tumor progression].Biomed Khim. 2018 Nov;64(6):472-486. doi: 10.18097/PBMC20186406472. Biomed Khim. 2018. PMID: 30632975 Review. Russian.
Cited by
-
Urokinase Plasminogen Activation System Modulation in Transformed Cell Lines.Int J Mol Sci. 2025 Jan 15;26(2):675. doi: 10.3390/ijms26020675. Int J Mol Sci. 2025. PMID: 39859388 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

