The Characterization and Modification of a Novel Bifunctional and Robust Alginate Lyase Derived from Marinimicrobium sp. H1
- PMID: 31547564
- PMCID: PMC6835848
- DOI: 10.3390/md17100545
The Characterization and Modification of a Novel Bifunctional and Robust Alginate Lyase Derived from Marinimicrobium sp. H1
Abstract
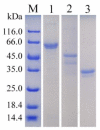
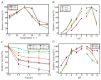
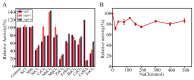
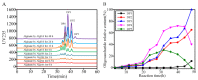
Alginase lyase is an important enzyme for the preparation of alginate oligosaccharides (AOS), that possess special biological activities and is widely used in various fields, such as medicine, food, and chemical industry. In this study, a novel bifunctional alginate lyase (AlgH) belonging to the PL7 family was screened and characterized. The AlgH exhibited the highest activity at 45 °C and pH 10.0, and was an alkaline enzyme that was stable at pH 6.0-10.0. The enzyme showed no significant dependence on metal ions, and exhibited unchanged activity at high concentration of NaCl. To determine the function of non-catalytic domains in the multi-domain enzyme, the recombinant AlgH-I containing only the catalysis domain and AlgH-II containing the catalysis domain and the carbohydrate binding module (CBM) domain were constructed and characterized. The results showed that the activity and thermostability of the reconstructed enzymes were significantly improved by deletion of the F5/8 type C domain. On the other hand, the substrate specificity and the mode of action of the reconstructed enzymes showed no change. Alginate could be completely degraded by the full-length and modified enzymes, and the main end-products were alginate disaccharide, trisaccharide, and tetrasaccharide. Due to the thermo and pH-stability, salt-tolerance, and bifunctionality, the modified alginate lyase was a robust enzyme which could be applied in industrial production of AOS.
Keywords: PL7 family; alginate lyase; alginate oligosaccharides; bifunctional; robust enzyme.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Inoue A. Characterization of PL-7 Family Alginate Lyases from Marine Organisms. Methods Enzymol. 2018;605:499–524. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

