The Interactivity between TGFβ and BMP Signaling in Organogenesis, Fibrosis, and Cancer
- PMID: 31547567
- PMCID: PMC6829314
- DOI: 10.3390/cells8101130
The Interactivity between TGFβ and BMP Signaling in Organogenesis, Fibrosis, and Cancer
Abstract
The Transforming Growth Factor beta (TGFβ) and Bone Morphogenic Protein (BMP) pathways intersect at multiple signaling hubs and cooperatively or counteractively participate to bring about cellular processes which are critical not only for tissue morphogenesis and organogenesis during development, but also for adult tissue homeostasis. The proper functioning of the TGFβ/BMP pathway depends on its communication with other signaling pathways and any deregulation leads to developmental defects or diseases, including fibrosis and cancer. In this review we explore the cellular and physio-pathological contexts in which the synergism or antagonism between the TGFβ and BMP pathways are crucial determinants for the normal developmental processes, as well as the progression of fibrosis and malignancies.
Keywords: BMPs; Non-SMAD signaling; SMAD pathway; TGFβ; adult homeostasis; cancer; development; disease; fibrosis; signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


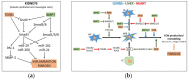

References
-
- Dituri F., Mancarella S., Cigliano A., Chieti A., Giannelli G. TGFβ as Multifaceted Orchestrator in HCC Progression: Signaling, EMT, Immune Microenvironment, and Novel Therapeutic Perspectives. Semin. Liver Dis. 2019;39:53–69. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

