The Impact of Lactoferrin on the Growth of Intestinal Inhabitant Bacteria
- PMID: 31547574
- PMCID: PMC6801499
- DOI: 10.3390/ijms20194707
The Impact of Lactoferrin on the Growth of Intestinal Inhabitant Bacteria
Abstract
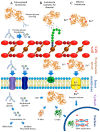
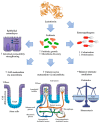
Lactoferrin (Lf) is an iron-binding milk glycoprotein that promotes the growth of selected probiotic strains. The effect of Lf on the growth and diversification of intestinal microbiota may have an impact on several issues, including (i) strengthening the permeability of the epithelial cell monolayer, (ii) favoring the microbial antagonism that discourages the colonization and proliferation of enteric pathogens, (iii) enhancing the growth and maturation of cell-monolayer components and gut nerve fibers, and (iv) providing signals to balance the anti- and pro-inflammatory responses resulting in gut homeostasis. Given the beneficial role of probiotics, this contribution aims to review the current properties of bovine and human Lf and their derivatives in in vitro probiotic growth and Lf interplay with microbiota described in the piglet model. By using Lf as a component in pharmacological products, we may enable novel strategies that promote probiotic growth while conferring antimicrobial activity against multidrug-resistant microorganisms that cause life-threatening diseases, especially in neonates.
Keywords: bifidobacteria; immunomodulation; intestinal homeostasis; lactobacilli; lactoferricin; lactoferrin; milk.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Nazir S.N.M., Yasmeen A., Usman S. Review study on lactoferrin: A multifunctional protein. Sky J. Food Sci. 2017;6:014–020.
-
- Berding K., Wang M., Monaco M.H., Alexander L.S., Mudd A.T., Chichlowski M., Waworuntu R.V., Berg B.M., Miller M.J., Dilger R.N., et al. Prebiotics and Bioactive Milk Fractions Affect Gut Development, Microbiota, and Neurotransmitter Expression in Piglets. J. Pediatr. Gastroenterol. Nutr. 2016;63:688–697. doi: 10.1097/MPG.0000000000001200. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

